Abstract
Specific Aim
Although the Department of Veterans Affairs (VA) has made significant organizational changes to improve diabetes care, diabetes self-management has received limited attention. The purpose of this study is to assess factors influencing diabetes self-management among veterans with poorly controlled diabetes.
Methods
Surveys were mailed to patients with type 2 diabetes and a HbA1c of 8% or greater who attended 1 of 2 VA Medical Centers in Washington State (n = 1,286). Validated survey instruments assessed readiness to change, self-efficacy, provider advice, and diabetes self-care practices.
Results
Our response rate was 56% (n = 717). Most respondents reported appropriate advice from physicians regarding physical activity, nutrition, and glucose monitoring (73%, 92%, and 98%, respectively), but many were not ready to change self-management behaviors. Forty-five percent reported non-adherence to medications, 42% ate a high-fat diet, and only 28% obtained either moderate or vigorous physical activity. The mean self-efficacy score for diabetes self-care was low and half of the sample reported readiness to change nutrition (52%) or physical activity (51%). Individuals with higher self-efficacy scores were more likely to adhere to medications, follow a diabetic meal plan, eat a lower fat diet, have higher levels of physical activity, and monitor their blood sugars (P < .001 for all).
Conclusions
Although veterans with poor diabetes control receive appropriate medical advice, many were not sufficiently confident or motivated to make and maintain self-management changes. Targeted patient-centered interventions may need to emphasize increasing self-efficacy and readiness to change to further improve VA diabetes outcomes.
Key Words: diabetes, diabetes self-management, veterans
INTRODUCTION
While the Department of Veterans Affairs (VA) has implemented many organizational changes to improve diabetes care,1–3 less emphasis has been placed on disease self-management. Although significant improvements in outcomes have been achieved, a large number of veterans still have poorly controlled diabetes. Because disease self-management including medication adherence, nutrition therapy, and physical activity is strongly related to disease control,4 information about current diabetes self-management practices is needed to guide quality improvement efforts.
Many factors influence diabetes self-management; however, most models have not been tested among veterans, a unique population with high rates of diabetes, comorbidity and disability.5–7 An individual’s “readiness to change,” their confidence in being able to make change (or self-efficacy), in addition to appropriate advice from medical providers, may impact diabetes self-management behavior (Fig. 1).8,9 Among nonveterans with diabetes, interventions designed to increase self-efficacy have improved quality of life, patient satisfaction, and glycemic control,10,11 and recent studies validate readiness to change as an important predictor of dietary behavior,12,13 physical activity,14–16 and improved glycemic control.17,18 The purpose of this study is to assess current levels of diabetes self-management and their association with self-efficacy, readiness to change, and provider advice among veterans with poorly controlled diabetes.
Figure 1.
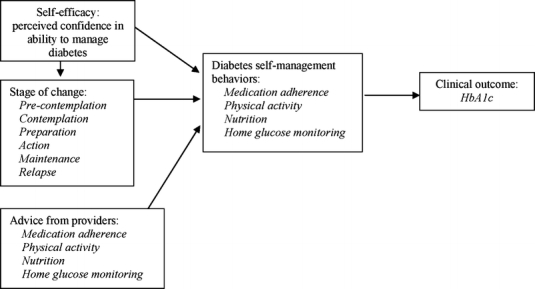
Conceptual model
METHODS
Study Design
We performed a mailed survey among patients with type 2 diabetes and an HbA1c value of 8% or greater who received primary care at two VA clinics in Washington State. Each patient was mailed an introductory letter, the 50-question survey, and a stamped postcard to return if they declined participation. After the first mailing, a second survey was mailed to nonresponders. Administrative databases were used to ascertain HbA1c, service-connected status, health care utilization, prescription refills, and race/ethnicity. This study was approved by the Institutional Review Board of the University of Washington.
Study Population
The diagnosis of diabetes was based on assignment of the diagnosis (ICD-9 codes 250, 357.2, 362.0, 366.4) during either an outpatient visit or inpatient admission.5,19 Eligibility criteria included enrollment in the VA primary care for more than 1 year; 2 or more clinic visits within the year before the study (June 30, 2003–June 30, 2004); and geographic proximity to two VA clinics in Washington state (zip codes 98001–98951). Patients under the age of 30 were assumed to have type 1 diabetes and were excluded from the study. Of 9,221 patients with diabetes at these two VA facilities, 1,340 (14.5%) were initially identified as eligible for study participation. Of those not eligible for the study (n = 7,881), 56.4% had HbA1c values of less than 8.0%, 21.6% did not live in the specified zip code range, 18.1% had less than 2 clinic visits in the previous year or were not enrolled in a primary care clinic, and 3.4% died during the study year.
Study Variables
The survey included information on demographics, smoking history, and self-reported health status,20 and used a previously validated measure of comorbidity among veterans.21 To measure self-efficacy, we used the Perceived Competence in Diabetes Scale, a validated 4-item scale that assessed respondent’s confidence in their ability to manage their diabetes care (Table 2).10 For each of the four components, the scale ranged from 1 (not at all true) to 7 (very true). For ease of interpretation, the total score was converted to 0–100.22 Readiness to change health behavior was assessed using validated questionnaires for physical activity and nutrition.23,24
Table 2.
Self-Efficacy, Stage of Change and Provider Advice for Disease Self-Management, n = 717
| Variables | Results | |
|---|---|---|
| Self-efficacy (perceived competence for diabetes care)* | Mean score | |
| Four components | 67.9 (SD ± 25.7); range 14.3–100 | |
| Confident in ability to manage diabetes | ||
| Capable of handling my diabetes | ||
| Able to do my own routine diabetes care | ||
| Able to meet the challenge of controlling my diabetes | ||
| Participant Stage of change† | Percentage (%) | |
| Physical activity | Precontemplative | 10 |
| Contemplative | 17 | |
| Preparation | 6 | |
| Action | 22 | |
| Maintenance | 29 | |
| Relapse | 16 | |
| Nutrition | Precontemplative | 10 |
| Contemplative | 23 | |
| Preparation | 8 | |
| Action | 22 | |
| Maintenance | 30 | |
| Relapse | 7 | |
| Provider/physician advice for | ||
| Physical activity | Daily low level exercise | 73 |
| 20 minutes exercise | 44 | |
| Fit exercise into daily routine | 43 | |
| Specific exercise program | 40 | |
| No advice reported | 27 | |
| Nutrition | Follow a low-fat meal plan | 66 |
| Follow complex carbohydrate diet | 59 | |
| Reduce calories | 58 | |
| Increase dietary fiber | 54 | |
| Eat 5 or more fruits and vegetables | 59 | |
| Limit intake of sweets | 81 | |
| No advice reported | 8 | |
| Provider assessed medication adherence | 69 | |
| Provider advised self-monitoring blood glucose | 98 | |
*Range of scores from 0–100, higher score with higher self-efficacy in managing diabetes.7
†The stages of change assessed are precontemplation: no intention of making a change; contemplation: considering change but not in the immediate future; preparation: solidifying commitment and planning for change; action: engaging in a new behavior, maintenance: sustaining the ongoing practice of a new behavior and relapse: previously engaged, but not currently practicing the health behavior.5
To assess levels of diabetes self-management, we used the Summary of Diabetes Self-Care Activities.25 Respondents reported on the frequency they performed recommended self-care activities over the past 7 days. Items from this scale assess provider advice regarding medication adherence, physical activity, nutrition, and smoking. Medication adherence was measured using a validated index by Choo et al.26 The impact of financial concerns on medication adherence was assessed using the questions from Piette et al.27
Physical activity level was assessed using the Physical Activity Scale for the Elderly (PASE).28 This scale measures total leisure, housework, and occupational activity through a weighted scoring of hours per activity in the previous 7 days. Scores range from 0 to 400, with a higher score signifying more physical activity. The PASE score has construct validity with health status measures and test–retest reliability among older individuals.28,29
The nutritional assessment included the Diet Habits Questionnaire (DHQ) and a short food frequency questionnaire.30,31 The DHQ has 21-items that assess five dimensions of low-fat dietary habits: substituting fat-modified food for high-fat foods, modifying meat to be lower in fat, avoiding fried food or fat as a flavoring, and replacing high-fat food with fruits or vegetables. These scales are reliable, sensitive to change, correlate with longer food frequency questionnaires among individuals with type 2 diabetes32 and can classify individuals as eating a high-fat diet.31
Data Analysis
We used bivariate analyses to assess the association between self-efficacy, stage of change, and provider advice with diabetes self-care behaviors. We used t-tests or analysis of variance (ANOVA) to determine the bivariate association of the self-efficacy score with medication adherence, physical activity, nutrition, glucose self-monitoring and stage of change. Multivariate regression analyses were used to determine the independent association of self-efficacy with self-care behaviors, controlling for age and comorbidity. Multivariate linear regression analysis was used to assess the independent relationship between diabetes self-care behaviors and HbA1c, controlling for age and comorbid disease. Analyses were performed using STATA, version 8.2 (StataCorp, College Station, TX).
RESULTS
A total of 1,340 surveys were mailed, of which 46 were returned with invalid addresses and 8 individuals were deceased. Of 1,286 eligible potential participants, 114 (9%) returned a postcard refusing participation, 717 (56%) sent back completed surveys, and 455 (35%) did not respond. There were no significant differences in demographics, race/ethnicity, glycemic control, health care utilization or service-connected disability between responders and nonresponders (data not shown).
Population Characteristics and Diabetes Self-Management Behaviors
There were significant levels of non-adherence to medications with one-fifth of respondents missing their medications 2 or more days per week (Table 1). Most respondents reported low levels of physical activity. The average physical activity score (PASE) was 99.6 (SD 73.4), significantly lower than scores reported in community-dwelling older populations.28,33 Although 67% of respondents reported following a diabetic meal plan, 42% of respondents were classified as having a high-fat diet.
Table 1.
Population Characteristics and Diabetes Self-Management Among Veterans with Type 2 Diabetes and Poor Glycemic Control, n = 717
| Variables | Results | |
|---|---|---|
| Demographics and medical comorbidity | Percentage (%) | |
| Male | 96 | |
| Age | 30–54 years | 22 |
| 55–64 years | 44 | |
| ≥65 years | 34 | |
| Comorbid conditions | COPD, asthma, or bronchitis | 26 |
| Myocardial infarction | 24 | |
| Congestive heart failure | 18 | |
| Stroke | 13 | |
| Cancer | 10 | |
| Smoking status | Current | 20 |
| Past | 56 | |
| Never | 23 | |
| Self-rated health | Excellent | 1 |
| Very Good | 9 | |
| Good | 33 | |
| Fair | 38 | |
| Poor | 19 | |
| Diabetes control | ||
| HbA1c % | ||
| Mean ± SD = 9.4 ± 1.5 | ||
| HbA1c ≥9%, n(%) | 358 (50) | |
| Diabetes self-management behaviors | ||
| Medication adherence | ||
| Highly adherent (missed medications 0 days per week) | 55 | |
| Moderately non-adherent (missed medications 1 day per week) | 24 | |
| Non-adherent (missed medications 2 or more days per week) | 21 | |
| Physical activity (prior week) | ||
| Walk outside your home | 81 | |
| Light physical activity | 33 | |
| Moderate physical activity | 16 | |
| Vigorous physical activity | 12 | |
| Isometric exercise | 30 | |
| Nutrition | ||
| Follow diabetic meal plan | 67 | |
| Ate ≥5 fruits/vegetables per day/past week | 14 | |
| Ate no fruits/vegetables per day/past week | 22 | |
| High-fat diet* | 42 | |
| Blood glucose self-monitoring | Mean ± SD | |
| Number of times per day monitored blood glucose | 2 ± 1 | |
| Number of days/week monitored blood glucose | 5 ± 2 | |
*Based on Dietary Habit Questionnaire31; column totals may vary due to rounding error.
Independent Variables: Self-Efficacy, Stage of Change, and Provider Advice
On average, respondents reported low levels of self-efficacy and were not ready to change lifestyle behaviors (Table 2). For both physical activity and diet, one-third of respondents were precontemplative or contemplating a change. The majority of respondents reported provider advice regarding daily, low-level exercise and appropriate recommendations for nutritional intake. Most participants reported that their health care provider asked about medication adherence (69%) and recommended self-monitoring blood glucose (98%).
Association of Self-Efficacy, Stage of Change, and Provider Advice with Diabetes Self-Management
Individuals with higher self-efficacy scores or who reported provider advice were more likely to be adherent to medications, walk for exercise, follow a diabetic meal plan, and eat a low-fat diet (Table 3). Individuals in an action or maintenance stage of change for nutrition had lower HbA1c levels (P < 0.001), were more likely to report provider advice regarding diet (P < 0.001), and had higher self-efficacy scores (P < 0.001) (Fig. 2). Individuals who relapsed with their diets had significantly higher HbA1c levels (P < 0.001) and lower self-efficacy scores (P < 0.001). Individuals in the relapse stage for physical activity had higher HbA1c levels (P < 0.05), were less likely to report provider advice regarding physical activity (P < 0.001), and had lower self-efficacy scores (P < 0.001).
Table 3.
Association of Diabetes Self-Management and Perceived Competence and Provider Advice, n = 717
| Self-management behavior | Perceived competence score (range 0–100) (mean score) | Received provider advice (%) |
|---|---|---|
| Medication adherence* | ||
| Highly adherent | 74.2 | 67 |
| Moderately non-adherent | 66.3 | 68 |
| Non-adherent | 47.8† | 76 |
| Physical activity | ||
| Walking for exercise | 69.8 | 82 |
| No walking | 53.9† | 65† |
| Nutrition | ||
| Follow diabetic meal plan | 70.5 | 97 |
| Does not follow diabetic meal pan | 60.0† | 83† |
| High-fat diet‡ | 59.1 | 90 |
| Lower fat diet | 72.4† | 94§ |
Figure 2.
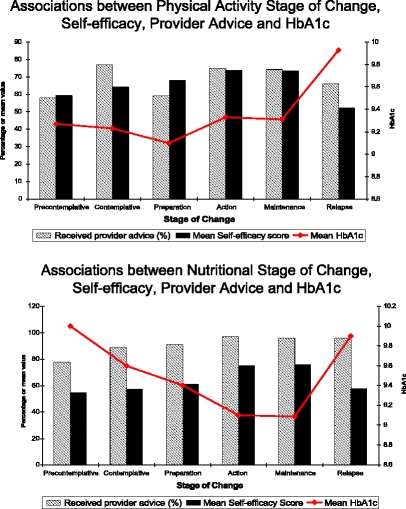
Stage of change, self-efficacy, provider advice and HbA1c
In multivariate models for each diabetes self-management practice, the self-efficacy score was independently associated with following a meal plan, physical activity score (PASE), adherence to medication, and daily glucose self-monitoring, controlling for age and comorbidity (data not shown). In a multivariate linear regression model controlling for age and comorbidity, non-adherence to medications was the strongest independent predictor for a higher HbA1c [Odds ratio (OR = 1.42, 95% C.I. = 1.31, 1.55]. Protective factors that were significantly associated with lower HbA1c levels included moderate to vigorous exercise (OR = 0.80, 95% C.I.=0.64, 0.99) and following a diabetic meal plan (OR = 0.71, 95% C.I. = 0.57, 0.89).
DISCUSSION
In our study of veterans with poor glycemic control, we found suboptimal diabetes self-management practices similar to levels reported in the nonveteran population.34–41 Despite appropriate provider advice, a significant number of respondents reported limited confidence in their ability to manage their diabetes and were not sufficiently motivated to make behavior changes. Although the majority of respondents perform home glucose monitoring, they may not take appropriate action with this information, as evidenced by their poor glycemic control.
Although the VA has been very successful at implementing many organizational features to improve diabetes care,2 less emphasis has been placed on supporting patient diabetes self-management. The VA has implemented an electronic medical record and electronic prescriptions, chronic disease registries, provider feedback, and decision support with national guidelines.1 Although there is evidence that the quality of care at the VA is high,3,42 diabetes outcomes are still not optimal. The VA quality improvement organization identifies diabetes self-management as a key element to improve clinical outcomes.43 Ours is one of the first studies to look specifically at potential barriers to veteran diabetes self-management in a high-risk population.
Studies of collaborative, patient-directed disease self-management programs among nonveterans have more favorable outcomes than traditional diabetes education programs.44,45 Self-efficacy theory and the stage of change model has been used as a framework to design interventions to increase physical activity and improve nutrition and glucose control among individuals with type 2 diabetes, but none, to our knowledge, have studied behavior change among veterans.12,15,17,46,47 Our findings that Hba1c is reduced among individuals in an action or maintenance stage of change and among those with higher levels of self-efficacy support the hypothesis that outcomes among veterans may be improved by focusing on these patient-related factors.
Our study has several limitations. The data are cross-sectional, and causality cannot be assumed. Data on medication adherence, nutritional intake, and physical activity were obtained by self-report and may be limited by recall and other biases. Although the survey did not collect data on type of medications, duration of diabetes, and several comorbid conditions, data is available from several national surveys. Most veterans with diabetes use oral medications (69%), 25% use insulin, and 6% do not use any medications.7 In a survey of 1,593 patients with diabetes in 14 VA facilities, 95.5% had type 2 diabetes, 37% had diabetes for 5 years or less, two-thirds had hypertension, and 22% reported depression.38 Ten percent of veterans with diabetes have comorbid renal disease.48 We do not have information about psychiatric comorbidity in our population, but given the high rates of depression and other psychiatric disorders among veterans, this may impact diabetes self-management.49,50 Our study population with poor glycemic control represented 15% of the veterans with diabetes at the two VA facilities; however, our surveyed population had similar demographics to the general veteran population with diabetes.5–7,34,51 Our study population was predominantly older men; thus, results may not apply to women or younger individuals. The results of this study may be generalizable to other veteran populations and other populations where the patient demographics are similar, such as males enrolled in Medicare.52
Although our population had available health care and diabetes education programs and received appropriate medical advice about diabetes self-management, veterans with poor glycemic control were found to be lacking in self-efficacy and were not appropriately motivated to make changes in health behaviors. The high level of medication non-adherence and the unhealthy lifestyles reported by our sample suggest that interventions aimed only at provider behavior may not be effective. Targeted patient-centered interventions, specifically to increase self-efficacy and readiness to change health behaviors, may be needed to achieve further gains in VA diabetes outcomes.
Acknowledgements
This project received funding from Health Services Research and Development, Department of Veterans Affairs, Diabetes QUERI (DIB 04-385). The views expressed in this article are those of the authors and do not represent the views of the Department of Veterans Affairs. This research was presented in part at the April 26–29, 2006 annual meeting of the Society for General Internal Medicine and the February 21--23, 2007 annual meeting of VA Health Services Research and Development. Thanks to Jane Emens for administrative support.
Potential Financial Conflicts of Interest None disclosed.
References
- 1.Jackson GL, Yano EM, Edelman D, Krein SL, Ibrahim MA, Carey TS, Lee SY, Hartmann KE, Dudley TK, Weinberger M. Veterans Affairs primary care organizational characteristics associated with better diabetes control. Am J Manag Care. Apr 2005;11(4):225–237. [PubMed]
- 2.Wagner EH, Glasgow RE, Davis C, Bonomi AE, Provost L, McCulloch D, Carver P, Sixta C. Quality improvement in chronic illness care: a collaborative approach. Joint Comm J Qual Improve. 2001;27(2):63–80. [DOI] [PubMed]
- 3.Kerr EA, Gerzoff RB, Krein SL, Selby JV, Piette JD, Curb JD, Herman WH, Marrero DG, Narayan KM, Safford MM, Thompson T, Mangione CM. Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: the TRIAD study. Ann Intern Med. Aug 17 2004;141(4):272–281. [DOI] [PubMed]
- 4.Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD, Purnell JQ, Wheeler M. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. Jan 2002;25(1):148–198. [DOI] [PubMed]
- 5.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. May 2004;27 Suppl 2:B10–21. [DOI] [PubMed]
- 6.Reiber GE, Boyko EJ, Maynard C, Koepsell TD, Pogach LM. Diabetes in the Department of Veterans Affairs. Diabetes Care. May 2004;27 Suppl 2:B1–2. [DOI] [PubMed]
- 7.Reiber GE, Koepsell TD, Maynard C, Haas LB, Boyko EJ. Diabetes in nonveterans, veterans, and veterans receiving Department of Veterans Affairs health care. Diabetes Care. May 2004;27 Suppl 2:B3–9. [DOI] [PubMed]
- 8.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. Sep–Oct 1997;12(1):38–48. [DOI] [PubMed]
- 9.Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;8:191–245. [DOI] [PubMed]
- 10.Williams G, Freedman Z, Deci E. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. October 1, 1998 1998;21(10):1644–1651. [DOI] [PubMed]
- 11.Anderson RM, Funnell MM, Butler PM, Arnold MS, Fitzgerald JT, Feste CC. Patient empowerment. Results of a randomized controlled trial. Diabetes Care. 1995;18(7):943–949. [DOI] [PubMed]
- 12.Jones H, Edwards L, Vallis TM, Ruggiero L, Rossi SR, Rossi JS, Greene G, Prochaska JO, Zinman B. Changes in diabetes self-care behaviors make a difference in glycemic control: the Diabetes Stages of Change (DiSC) study. Diabetes Care. Mar 2003;26(3):732–737. [DOI] [PubMed]
- 13.Vallis M, Ruggiero L, Greene G, Jones H, Zinman B, Rossi S, Edwards L, Rossi JS, Prochaska JO. Stages of change for healthy eating in diabetes: relation to demographic, eating-related, health care utilization, and psychosocial factors. Diabetes Care. May 2003;26(5):1468–1474. [DOI] [PubMed]
- 14.Plotnikoff RC, Brez S, Hotz SB. Exercise behavior in a community sample with diabetes: understanding the determinants of exercise behavioral change. Diabetes Educ. May–Jun 2000;26(3):450–459. [DOI] [PubMed]
- 15.Kirk AF, Higgins LA, Hughes AR, Fisher BM, Mutrie N, Hillis S, MacIntyre PD. A randomized, controlled trial to study the effect of exercise consultation on the promotion of physical activity in people with Type 2 diabetes: a pilot study. Diabet Med. Nov 2001;18(11):877–882. [DOI] [PubMed]
- 16.Natarajan S, Clyburn EB, Brown RT. Association of exercise stages of change with glycemic control in individuals with type 2 diabetes. Am J Health Promot. Sep–Oct 2002;17(1):72–75, ii. [DOI] [PubMed]
- 17.O’Connor PJ, Asche SE, Crain AL, Rush WA, Whitebird RR, Solberg LI, Sperl-Hillen JM. Is patient readiness to change a predictor of improved glycemic control? Diabetes Care. Oct 2004;27(10):2325–2329. [DOI] [PubMed]
- 18.Peterson KA, Hughes M. Readiness to change and clinical success in a diabetes educational program. J Am Board Fam Pract. Jul–Aug 2002;15(4):266–271. [PubMed]
- 19.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. Nov–Dec 1999;14(6):270–277. [DOI] [PubMed]
- 20.Ware J, Snow K, Kosinski M, Gandek B. SF-36 Health Survey. Manual and Interpretation Guide.: The Health Institute, New England Medical Center, Boston, MA; 1993.
- 21.Fan VS, Au D, Heagerty P, Deyo RA, McDonell MB, Fihn SD. Validation of case-mix measures derived from self-reports of diagnoses and health. J Clin Epidemiol. Apr 2002;55(4):371–380. [DOI] [PubMed]
- 22.Heisler M, Vijan S, Anderson RM, Ubel PA, Bernstein SJ, Hofer TP. When do patients and their physicians agree on diabetes treatment goals and strategies, and what difference does it make? J Gen Intern Med. Nov 2003;18(11):893–902. [DOI] [PMC free article] [PubMed]
- 23.CDC. Project PACE. Physicians Manual. Atlanta, Georgia: Centers for Disease Control, Cardiovascular Health Branch; 1992.
- 24.Kristal AR, Glanz K, Curry SJ, Patterson RE. How can stages of change be best used in dietary interventions? J Am Diet Assoc. Jun 1999;99(6):679–684. [DOI] [PubMed]
- 25.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. Jul 2000;23(7):943–950. [DOI] [PubMed]
- 26.Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, Canning C, Platt R. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. Sep 1999;37(9):846–857. [DOI] [PubMed]
- 27.Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care. Feb 2004;27(2):384–391. [DOI] [PubMed]
- 28.Washburn R, Smith K, Jette A, Janney C. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol. 1993;46:153–162. [DOI] [PubMed]
- 29.Washburn R, McAuley E, Katula J, Mihalko S, Boileau R. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. Jul 1999;52(7):643–651. [DOI] [PubMed]
- 30.Kristal AR, Shattuck AL, Henry HJ. Patterns of dietary behavior associated with selecting diets low in fat: reliability and validity of a behavioral approach to dietary assessment. J Am Diet Assoc. Feb 1990;90(2):214–220. [PubMed]
- 31.Shannon J, Kristal AR, Curry SJ, Beresford SA. Application of a behavioral approach to measuring dietary change: the fat- and fiber-related diet behavior questionnaire. Cancer Epidemiol Biomark Prev. May 1997;6(5):355–361. [PubMed]
- 32.Glasgow RE, Perry JD, Toobert DJ, Hollis JF. Brief assessments of dietary behavior in field settings. Addict Behav. Mar–Apr 1996;21(2):239–247. [DOI] [PubMed]
- 33.Chad KE, Reeder BA, Harrison EL, Ashworth NL, Sheppard SM, Schultz SL, Bruner BG, Fisher KL, Lawson JA. Profile of physical activity levels in community-dwelling older adults. Med Sci Sports Exerc. Oct 2005;37(10):1774–1784. [DOI] [PubMed]
- 34.Heisler M, Smith DM, Hayward RA, Krein SL, Kerr EA. How well do patients’ assessments of their diabetes self-management correlate with actual glycemic control and receipt of recommended diabetes services? Diabetes Care. Mar 2003;26(3):738–743. [DOI] [PubMed]
- 35.Nelson KM, Rieber G, Boyko EJ. Diet and exercise among adults with type 2 diabetes; Data from NHANES III. Diabetes Care. 2002;25:1722–1728. [DOI] [PubMed]
- 36.Eeley EA, Stratton IM, Hadden DR, Turner RC, Holman RR. UKPDS 18: Estimated dietary intake in type 2 diabetic patients randomly allocated to diet, sulphonylurea or insulin therapy. UK Prospective Diabetes Study Group. Diabet Med. Jul 1996;13(7):656–662. [DOI] [PubMed]
- 37.Murata GH, Shah JH, Duckworth WC, Wendel CS, Mohler MJ, Hoffman RM. Food frequency questionnaire results correlate with metabolic control in insulin-treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study. J Am Diet Assoc. Dec 2004;104(12):1816–1826. [DOI] [PubMed]
- 38.Reiber GE, Au D, McDonell M, Fihn SD. Diabetes quality improvement in Department of Veterans Affairs Ambulatory Care Clinics: a group-randomized clinical trial. Diabetes Care. May 2004;27 Suppl 2:B61–68. [DOI] [PubMed]
- 39.Glasgow RE, Ruggiero L, Eakin EG, Dryfoos J, Chobanian L. Quality of life and associated characteristics in a large national sample of adults with diabetes. Diabetes Care. Apr 1997;20(4):562–567. [DOI] [PubMed]
- 40.Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with Type 2 diabetes: a retrospective cohort study. Diabet Med. Apr 2002;19(4):279–284. [DOI] [PubMed]
- 41.Boccuzzi SJ, Wogen J, Fox J, Sung JC, Shah AB, Kim J. Utilization of oral hypoglycemic agents in a drug-insured U.S. population. Diabetes Care. Aug 2001;24(8):1411–1415. [DOI] [PubMed]
- 42.Nelson KM, Chapko MK, Reiber G, Boyko EJ. The association between health insurance coverage and diabetes care; data from the 2000 Behavioral Risk Factor Surveillance System. Health Serv Res. Apr 2005;40(2):361–372. [DOI] [PMC free article] [PubMed]
- 43.QUERI Fact Sheet: Diabetes Mellitus. VA QUERI (Quality Enhancement Research Initiative), June 2006, Ann Arbor, MI. Available at: http://www.hsrd.research.va.gov/publications/internal/dm_factsheet.pdf. Accessed Oct. 3, 2006.
- 44.Norris S, Engelgau MM, Venkay Narayan KM. Effectiveness of Self-Management training in type 2 diabetes. A systematic review of randomized controlled trials. Diabetes Care. 2001;24(3):561–587. [DOI] [PubMed]
- 45.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. Nov 20 2002;288(19):2469–2475. [DOI] [PubMed]
- 46.Kim CJ, Hwang AR, Yoo JS. The impact of a stage-matched intervention to promote exercise behavior in participants with type 2 diabetes. Int J Nurs Stud. Nov 2004;41(8):833–841. [DOI] [PubMed]
- 47.Parchman ML, Arambula-Solomon TG, Noel PH, Larme AC, Pugh JA. Stage of change advancement for diabetes self-management behaviors and glucose control. Diabetes Educ. Jan–Feb 2003;29(1):128–134. [DOI] [PubMed]
- 48.Young BA, Pugh JA, Maynard C, Reiber G. Diabetes and renal disease in veterans. Diabetes Care. May 2004;27 Suppl 2:B45–49. [DOI] [PubMed]
- 49.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160(21):3278–3285. [DOI] [PubMed]
- 50.Kazis LE, Miller DR, Clark J, Skinner K, Lee A, Rogers W, Spiro A, 3rd, Payne S, Fincke G, Selim A, Linzer M. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626–632. [DOI] [PubMed]
- 51.Thompson W, Wang H, Xie M, Kolassa J, Rajan M, Tseng CL, Crystal S, Zhang Q, Vardi Y, Pogach L, Safford MM. Assessing quality of diabetes care by measuring longitudinal changes in hemoglobin a1c in the veterans health administration. Health Serv Res. Dec 2005;40(6 Pt 1):1818–1835. [DOI] [PMC free article] [PubMed]
- 52.Centers for Medicare & Medicaid Services. Medicare Current Beneficiary Survey. 2002. Available at: http://www.cms.hhs.gov/apps/mcbs/. Accessed January 11, 2006.


