Abstract
BACKGROUND
Patients and providers may be reluctant to escalate to insulin therapy despite inadequate glycemic control.
OBJECTIVES
To determine the proportion of patients attaining and maintaining glycemic targets after initiating sulfonylurea and metformin oral combination therapy (SU/MET); to assess insulin initiation among patients failing SU/MET; and to estimate the glycemic burden incurred, stratified by whether HbA1c goal was attained and maintained.
DESIGN
Longitudinal observational cohort study.
SUBJECTS
Type 2 diabetes patients, 3,891, who newly initiated SU/MET between 1 January 1996 and 31 December 2000.
MEASUREMENTS
Subjects were followed until insulin was added, health plan disenrolment, or until 31 December 2005. We calculated the number of months subjects continued SU/MET therapy alone, in total, and during periods of inadequate glycemic control; the A1C reached during those time periods; and total glycemic burden, defined as the estimated cumulative monthly difference between measured A1C and 8%.
RESULTS
During a mean follow-up of 54.6 ± 28.6 months, 41.9% of the subjects added insulin, and 11.8% received maximal doses of both oral agents. Over half of SU/MET patients attained but failed to maintain A1C of 8%, yet continued SU/MET therapy for an average of nearly 3 years, sustaining glycemic burden equivalent to nearly 32 months of A1C levels of 9%. Another 18% of patients never attained the 8% goal with SU/MET, yet continued that therapy for an average of 30 months, reaching mean A1C levels of 10%.
CONCLUSIONS
Despite inadequate glycemic control, a minority of patients added insulin or maximized oral agent doses, thus, incurring substantial glycemic burden on SU/MET. Additional studies are needed to examine the benefits of rapid titration to maximum doses and earlier initiation of insulin therapy.
KEY WORDS: insulin, glycemic control, oral combination therapy
INTRODUCTION
Type 2 diabetes is a progressive disease that requires the ongoing intensification of pharmacotherapy to achieve and maintain glycemic control.1 The micro- and probable macro-vascular benefits of glycemic control are now well documented.2–4 When lifestyle modification alone can no longer maintain desired glycemic targets and clinicians and patients decide to begin drug therapy, sulfonylurea (SU) or metformin monotherapy are typically the first-line agents of choice.5 These agents are frequently used in combination after monotherapy fails, but the success of SU/metformin combination therapy (SU/MET) is often short-lived,6 with HbA1c (A1C) escalation resuming as early as 6 months after SUs are added to metformin.7
Because of progressive deficits in insulin secretion, patients often need insulin supplementation to achieve good glycemic control.8 A recent consensus statement from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommended insulin as the next therapy when combination metformin/sulfonylurea treatment does not result in goal glycemia.9 Prior studies have consistently demonstrated that fewer than half of patients with inadequate glycemic control intensify their antihyperglycemic therapy, a concept known as “clinical inertia”.10–12 This inertia may be particularly problematic when insulin is the next therapeutic option. Patients are often reluctant to initiate insulin, perhaps because of an aversion to insulin injections, a belief that they had failed proper diabetes self-management, a concern that insulin would restrict their lives, or other reasons.13 Clinicians can also be reluctant to initiate insulin.14 This patient and provider reluctance may lead to long periods of unnecessarily high A1C, or “glycemic burden”. Brown et al.15 recently described glycemic burden among subjects receiving SU/MET, but that study was limited to patients whose therapy was escalated by adding insulin or another oral agent. To our knowledge, no study to date has attempted to quantify the glycemic burden of SU/MET among patients who do and do not escalate pharmacotherapy. Our objectives were to determine the proportion of patients attaining and maintaining glycemic targets while receiving sulfonylurea and metformin oral combination therapy (SU/MET), to assess insulin initiation among patients failing SU/MET, and to estimate the glycemic burden incurred, stratified by whether HbA1c goal was attained and maintained.
RESEARCH DESIGN AND METHODS
The current study was conducted within Kaiser Permanente Northwest (KPNW), a not-for-profit, group-model health maintenance organization (HMO) that provides comprehensive, prepaid coverage to about 470,000 members. KPNW uses electronic health care utilization data to track and facilitate operations. An electronic medical record, in use since 1996, allows the attending clinician to record as many as 20 ICD-9-CM coded diagnoses at each ambulatory patient contact and up to nine discharge diagnoses for inpatient hospital admissions. An electronic problem list, also coded in ICD-9-CM, is available to the clinician at each contact. A single regional laboratory performs all KPNW laboratory tests, and the results are stored in a searchable database. A pharmacy is located in each medical office, and most members have a pharmacy benefit, helping to ensure complete capture of pharmaceutical dispenses. In the current study, 96% of subjects had a pharmacy benefit.
KPNW employs evidence-based guidelines to assist clinicians in their treatment decisions. During the course of this study, the antihyperglycemic therapy guideline algorithm recommended SU as the first-line agent, followed by addition of metformin, when necessary, and then insulin therapy when A1C was greater than 8%. From all KPNW members with type 2 diabetes (multiple ICD-9-CM diagnoses of 250.xx), we selected all 5,300 who newly initiated SU/MET between 1 January 1996 and 31 December 2000 (by adding one agent to an ongoing regimen or by simultaneously initiating both drugs) and who maintained SU/MET for at least 6 months. We excluded 900 subjects who did not have at least 6 months of health plan eligibility before and after SU/MET initiation. To focus the study on the recommended therapeutic progression from SU/MET to insulin, we excluded 414 subjects who received a third oral agent before insulin. We also excluded 95 subjects who did not have at least one A1C measurement during both the 6-month period before and the 6-month period after SU/MET initiation. Applying all of these criteria resulted in a final analysis sample of 3,891. Our primary end point was the addition of insulin to the patient’s treatment regimen. We followed subjects until insulin was added, health plan eligibility ended (because of disenrolment or death), or until 31 December 2005.
We examined all A1C measurements (Diamat assay, Bio-Rad, Hercules, CA) recorded in the KPNW regional laboratory database during the observation period (which corresponded to use of SU/MET alone). During the observation period, the recommended A1C level for therapeutic action was 8%. Using this level as the A1C goal, we calculated three measures of the degree to which patients failed to meet the goal. First, we calculated the number of months subjects spent above goal before initiating insulin therapy (or end of follow-up). Second, we observed the level of A1C reached before insulin initiation (or end of follow-up). Finally, we estimated overall glycemic burden as defined by Brown and colleagues—the cumulative amount by which A1C exceeded the 8% treatment goal. This method sums the difference between measured or interpolated A1C and goal over all observed months (one unit of glycemic burden is equivalent to 1 month of A1C at 9%, or 10 months of A1C at 8.1%).15 The result is expressed in “A1C months”.
We stratified patients into three groups: (1) those who achieved and maintained A1C goal for the entire observation period; (2) those who initially achieved but failed to maintain goal; and (3) those who never attained goal. By definition, subjects who attained and maintained A1C goal spent no time above goal after attaining it, but could still accumulate glycemic burden before the goal was attained. For those who initially achieved but did not maintain goal, we calculated months above goal from the first date the A1C was above goal (after previously being below goal). Among subjects who never attained the goal, months above goal equaled total observation months. To control for differences between strata that might affect the decision to initiate insulin, we constructed a Cox regression model of time-to-insulin-addition, adjusting for age, sex, diabetes duration, presence of cardiovascular disease, congestive heart failure, micro- or macroalbuminuria, body mass index, change in body weight, SU dose, metformin dose, SU and metformin adherence, number of A1C tests, and level of last A1C during observation. For subjects who either failed to maintain goal or never attained goal, we estimated the time to insulin addition with PROC LIFETEST, using a Kaplan–Meier plot to display the proportion of patients that added insulin over time.
Using electronic medication dispensing records, we ascertained the dose of each SU and metformin prescription. Glyburide was the SU dispensed 98% of the time. When an SU other than glyburide was used, we converted the daily dose of the prescribed SU to “glyburide equivalents” based on maximum doses of each agent.16 We estimated adherence to each of the medications using the medicine possession ratio, calculated as the total days supply of dispensed medications divided by the number of days of observation.17 Age and sex were extracted from membership records. We calculated body mass index from weight and height measurements taken before SU/MET initiation and calculated weight change based on the last weight measured during observation. From the electronic medical records, we identified presence of cardiovascular disease or congestive heart failure using the following ICD-9-CM codes: myocardial infarction, 410.xx; stroke, 430.xx–432.xx, 434.xx–436.xx, 437.1; other atherosclerotic cardiovascular disease, 411.1, 411.8, 413.xx, 414.0, 414.8, 414.9, 429.2; congestive heart failure, 428.xx. From laboratory data, we identified subjects with microalbuminuria (albumin excretion rate between 30 and 300 mg/day) or macroalbuminuria (albumin excretion rate > 300 mg/day or serum creatinine > 1.5 mg/dL or 24-hour urine protein > 165 mg/day).
All analyses were conducted using SAS statistical software version 8.2 (SAS Institute, Cary, NC). The study was reviewed and approved by the Institutional Review Board of Kaiser Permanente’s Center for Health Research.
RESULTS
Subjects were followed for a mean of 54.6 ± 28.6 months. Nearly one-fifth (18.1%) of subjects failed to attain the A1C goal of 8%, while just 24.3% reached and maintained that goal (Table 1). Patients who never attained A1C < 8% were significantly younger (53.8 ± 11.7 years) than those who maintained A1C < 8% (61.9 ± 11.9 years, P < .001) and those who attained but failed to maintain the 8% goal (59.2 ± 11.5 years, P < .001). All groups, on average, lost weight. However, patients who maintained goal lost 12.2 ± 19.1 lbs, those who did not maintain goal lost 9.9 ± 18.5 lbs, and those who never attained goal lost 6.9 ± 16.4 pounds (P < .001 all comparisons). Last observed doses of both metformin and SU were significantly lower among those who maintained goal than among those who never attained or failed to maintain goal. Adherence to SU as measured by the possession ratio was not significantly different across the three groups. However, metformin adherence (possession ratio) was best among those who maintained goal (0.78 ± 0.26), lower among those who attained but did not maintain goal (0.75 ± 0.26), and worst among those who never attained goal (0.70 ± 0.30, P < .001 all comparisons).
Table 1.
Subject Characteristics by Whether A1C Goal of 8% was Attained or Maintained
| Characteristics | Maintained 8% goal | Attained, did not maintain 8% goal | Never attained 8% goal |
|---|---|---|---|
| Number (%) of subjects | 944 (24.3%) | 2,241 (57.6%) | 706 (18.1%) |
| Age at SU/MET initiation* | 61.9 (11.9) | 59.2 (11.5) | 53.8 (11.7) |
| Percent (%) female | 51.1% | 49.0% | 47.2% |
| Diabetes duration (years) | 4.2 (3.2) | 4.3 (3.1) | 4.0 (2.8) |
| Body mass index at SU/MET initiation | 34.2 (7.6) | 34.2 (7.3) | 34.9 (7.5) |
| Weight change (pounds) at end offollow-up* | −12.2 (19.1) | −9.9 (18.5) | −6.9 (16.4) |
| Presence of cardiovascular disease* | 30.1% | 28.1% | 14.9% |
| Presence of congestive heart failure* | 15.4% | 13.8% | 7.7% |
| Presence of micro/macroalbuminuria* | 37.6% | 43.2% | 47.9% |
| Mean dose of last SU dispense (Glyburide equivs)† | 11.3 (7.8) | 13.8 (7.5) | 14.4 (7.3) |
| Distribution of last SU dose* | |||
| <5 mg/day | 13.6% | 5.9% | 3.4% |
| 5–9.9 mg/day | 21.1% | 12.4% | 9.8% |
| 10–14.9 mg/day | 32.5% | 33.3% | 37.3% |
| 15–19.9 mg/day | 7.2% | 9.8% | 9.2% |
| ≥20 mg/day | 25.6% | 38.6% | 40.4% |
| Sulfonylurea possession ratio | 0.79 (0.27) | 0.82 (0.24) | 0.81 (0.26) |
| Mean dose of last metformin dispense (mg)† | 1,675 (754) | 1,913 (677) | 1,865 (799) |
| Distribution of last metformin dose*: | |||
| <1,000 mg/day | 9.2% | 4.6% | 7.4% |
| 1,000–1,499 mg/day | 22.8% | 13.5% | 15.4% |
| 1,500–1,999 mg/day | 25.0% | 19.6% | 21.0% |
| 2,000–2,499 mg/day | 23.6% | 33.3% | 27.9% |
| ≥2,500 mg/day | 19.4% | 29.1% | 28.3% |
| Metformin possession ratio* | 0.78 (0.26) | 0.75 (0.26) | 0.70 (0.30) |
| Added insulin* | 18.1% | 43.9% | 67.6% |
Numbers shown are means (standard deviations) or percentages
*Groups differ, P < .001
†Maintained 8% goal differs from other two groups, P < 0.001
Subjects who attained A1C goal had similar mean A1C values before and immediately after initiating SU/MET, regardless of whether they maintained the goal (Table 2). Those who maintained the goal had a mean A1C of 8.8 ± 1.6% before and 7.8 ± 1.4% after SU/MET; the corresponding means were 9.1 ± 1.4% and 8.1 ± 1.3% among those who failed to maintain the goal A1C. Those who never attained 8% had mean A1C levels of 10.3 ± 1.7% before and 9.9 ± 1.4% after initiating SU/MET (P < .001, compared to both groups). Subjects who maintained an A1C < 8% took longer to attain the goal than those who failed to maintain it (10.3 ± 14.2 vs 9.2 ± 11.6 months, P < .022), but maintainers continued below 8% for over twice as long (44.6 ± 29.8 vs 17.1 ± 15.3 months, P < .001). In all groups, patients averaged at least 2 A1C measurements per year. Because patients who never attained goal were more likely to add insulin, they had the shortest total follow-up time, but incurred over twice the glycemic burden (63.9 ± 65.6 A1C months) compared to patients who attained but did not maintain the goal (31.8 ± 40.1 A1C months, P < .001).
Table 2.
Mean (Standard Deviation) A1C, Months Above and Below 8% Goal, and Glycemic Burden, by Whether Goal was Attained and Maintained
| Parameters | Maintained 8% goal | Attained, did not maintain 8% goal | Never attained 8% goal |
|---|---|---|---|
| Mean HbA1c Levels | |||
| Before SU/MET* | 8.8% (1.6%) | 9.1% (1.4%) | 10.3% (1.7%) |
| First HbA1c After SU/MET* | 7.8% (1.4%) | 8.1% (1.3%) | 9.9% (1.4%) |
| Best HbA1c during SU/MET* | 6.3% (0.7%) | 6.7% (0.7%) | 9.2% (1.2%) |
| Mean HbA1c during SU/MET* | 7.2% (0.8%) | 8.1% (0.8%) | 10.0% (1.3%) |
| Last HbA1c of follow-up* | 6.8% (0.7%) | 8.4% (1.6%) | 10.3% (1.6%) |
| Mean number of HbA1c tests per year | 2.1 (0.9) | 2.3 (1.0) | 2.0 (1.3) |
| Mean months | |||
| Until goal was attained | 10.3 (14.2) | 9.2 (11.6) | – |
| Below goal once it was attained* | 44.6 (29.8) | 17.1 (15.3) | – |
| Above goal after (if) attaining it | – | 35.8 (25.5) | – |
| Total Follow-up* | 54.9 (28.8) | 62.1 (26.3) | 30.1 (20.8) |
| Mean Glycemic Burden*† | 11.1 (28.8) | 31.8 (40.1) | 63.9 (65.6) |
*Groups differ, P < .001
†One unit of burden equals 1 month of HbA1c at 9%, or 10 months at 8.1%
Patients who never attained the goal were over four times more likely to add insulin than those who maintained goal (Table 3; HR 4.60, 95% CI 3.65−5.79, P < .0001). However, after accounting for other factors, subjects who attained but did not maintain A1C < 8% were not significantly more likely to initiate insulin than those who maintained the goal. Higher doses of SU but lower doses of metformin were predictive of insulin addition, as was better adherence to each drug. Higher body mass index at SU/MET initiation and greater weight gain (or less loss) predicted insulin use. Women were 13% more likely to initiate insulin (1.13, 1.02–1.26, P = .023). Age and comorbidities were not significant factors.
Table 3.
Predictors of Insulin Addition from Multivariate Cox Proportional Hazards Regression Model
| Parameters | Hazard ratio (95% CI) | P value |
|---|---|---|
| Maintained HbA1c < 8% (referent) | 1.00 | – |
| Attained, did not maintain 8% | 1.15 (0.96–1.01) | 0.131 |
| Never attained 8% | 4.60 (3.65–5.79) | <0.0001 |
| Age (per 5 years) | 0.99 (0.96–1.01) | 0.308 |
| Female sex | 1.13 (1.02–1.26) | 0.023 |
| Duration of diabetes | 0.97 (0.96–0.99) | 0.005 |
| Cardiovascular disease | 0.95 (0.83–1.08) | 0.415 |
| Congestive heart failure | 0.88 (0.74–1.04) | 0.119 |
| Micro-/macroalbuminuria | 1.02 (0.92–1.14) | 0.663 |
| Baseline body mass index | 1.02 (1.01–1.03) | 0.0001 |
| Weight change during follow-up (per 5 lbs) | 1.08 (1.06–1.09) | <0.0001 |
| Daily dose of sulphonylurea (per 5 mg of Glyburide) | 1.06 (1.03–1.10) | 0.001 |
| Daily dose of metformin (per 500 mg) | 0.93 (0.89–0.98) | 0.002 |
| Mean HbA1c tests per year of follow-up | 1.75 (1.68–1.83) | <0.0001 |
| Last HbA1c of follow-up | 1.27 (1.23–1.31) | <0.0001 |
| Sulfonylurea possession ratio | 1.95 (1.50–2.55) | <0.0001 |
| Metformin possession ratio | 1.86 (1.47–2.34) | <0.0001 |
Figure 1 displays Kaplan–Meier estimates of time to insulin addition for those who attained but failed to maintain 8% (calculated from the point at which goal was no longer maintained) and for those who never attained 8%. By 24 months, about 50% of subjects who never attained goal had added insulin. In contrast, it took about 60 months for 50% of those who attained but did not maintain goal to add insulin (log-rank test, P < .0001).
Figure 1.
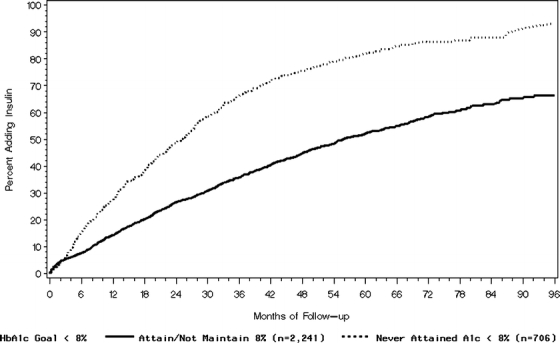
Kaplan–Meier analysis of time to insulin addition.
DISCUSSION
In this retrospective longitudinal cohort study, we found that over 80% of the 3,891 type 2 diabetes patients newly initiating SU/MET achieved the A1C goal of 8%. However, most patients who succeeded in reaching that goal were unable to maintain it. Despite A1C levels in excess of the then-recommended level for therapeutic action and well in excess of the currently recommended level of 7%,18 patients continued SU/MET therapy alone for an average of nearly 3 years. We calculated months above target from the test date of the first elevated A1C, likely resulting in an underestimate of the actual time above goal. Because of this long delay, patients sustained substantial glycemic burden equivalent to nearly 32 months of A1C levels of 9%. Moreover, 18% of patients never attained the 8% goal with SU/MET, yet continued that therapy for an average of 30 months while reaching average A1C levels of 10%. These A1C levels 1–2% persistently above goal may result in potentially avoidable diabetic complications. In the United Kingdom Prospective Diabetes Study (UKPDS), for example, each 1% reduction in mean A1C was associated with a 21% reduction in any diabetes-related end point.19
Although previous studies in this and other study settings have demonstrated that clinical inertia is common in the treatment of hyperglycemia,6,10–12,15 to our knowledge, the current study is the first to quantify clinical inertia over several years. Our study focused on SU/MET patients whose next therapeutic step (according to KPNW internal guidelines) was insulin, a population in which clinical inertia may be particularly problematic. Previous studies have described the concept of “psychological insulin resistance,” in which patients and providers are reluctant to initiate subcutaneous insulin therapy.20–24 Our results suggest that such reluctance may lead to long periods of elevated glycemic levels that put patients at increased risk for diabetes complications. We could not ascertain the reasons for delaying insulin initiation in our data. Although there are legitimate clinical reasons for not initiating insulin, such as advanced age or heart failure, these variables did not predict insulin initiation in our multivariate model. Concern about weight gain might also deter insulin use, but in our model, higher baseline BMI and greater weight gain predicted insulin initiation, confirming the value of weight loss in reducing hyperglycemia. Thus, it appears that unmeasured variables such as psychological insulin resistance likely contribute to the delay of therapy intensification.
Reluctance to initiate insulin would not be the only explanation for why patients continue SU/MET alone despite inadequate glycemic control. For example, we found that less than half of patients were receiving maximum doses of either SU or metformin during their SU/MET, suggesting that dose adjustments within their current therapy could improve glycemic control. Mean doses of SU and metformin, however, were higher in subjects who either failed to maintain or who never attained goal than among those who attained and maintained goal. After multivariate adjustment, higher doses of metformin did slow progression to insulin, but higher doses of SU predicted insulin initiation. The therapeutic benefit of increasing doses beyond those we report could not be determined from these data.
The lower doses in those who achieved and maintained an A1C < 8% may reflect the greater difficulty patients have achieving the goal as their A1C levels become higher. As our data show, patients who never attained goal had substantially higher A1C levels before initiating SU/MET compared to those who attained goal. These results are consistent with recent reports of secondary failure of SU and metformin monotherapy conducted in the same setting.25,26 Those studies also demonstrated that the duration of an individual agents’ success was inversely related to the A1C reduction achieved in the first year of use. We observed a similar pattern in the current study—subjects who attained the A1C goal reduced their A1C by about one percentage point at the first measurement after SU/MET initiation, while those who failed to reach goal had mean A1C levels still near 10%. Adherence to metformin might explain some of the difference in success, as it was significantly greater among those who maintained goal compared to those who never attained 8%. However, after controlling for other factors, better adherence to both SU and metformin predicted insulin initiation. This finding may reflect a greater willingness among adherent patients to start insulin therapy, or a greater sense that these patients had maximized the benefit of SU/MET. In any case, SU/MET is not a long-term therapy solution for most patients, regardless of dose or adherence. Given that three-fourths of our study subjects never attained or failed to maintain A1C goal, our results suggest that patients will most benefit from the new guidelines that lowered A1C goal with more aggressive intensification of glycemic control regimens, perhaps by initiating insulin sooner.
Another therapeutic option when SU/MET fails is the addition of other oral agents, such as thiazolidinediones. The ADA/EASD consensus statement offers this option when A1C is close to goal,9 but these drugs are not widely used in our study setting. We chose to exclude the 414 potential subjects who initiated a third oral agent from our study because they or their clinicians did not follow the predominant practice pattern in our setting, and therefore, did not represent most SU/MET patients.
Our results are not likely specific to the study setting. Previous research has shown that members of the study HMO receive more guideline-adherent care and achieve lower-than-average risk factors levels, with mean A1C levels of 7.5%.6,27,28 Our subjects were not a neglected subset of the HMO—they averaged over two A1C measurements per year and nearly 10 measurements during the study period. In addition, our study likely understates the glycemic burden accumulated during SU/MET because we could not follow patients beyond the end of observation. Thus, the months over A1C goal that we observed is the lower limit incurred preceding therapeutic action. Our findings are especially relevant now that the recent ADA/EASD consensus statement recommends insulin as the third line agent when A1C is greater than 7% with SU/MET.9 Furthermore, a recent observational study conducted in New England concluded that use of multiple oral antidiabetic agents may serve as a marker for uncontrolled diabetes.29 Considering that the Diabetes Attitudes, Wishes, and Needs (DAWN) study recently demonstrated that resistance to injected insulin therapy is an international phenomenon,14 patients worldwide may be experiencing glycemic burden levels at least similar to and probably greater than those we report. However, it is important to note that we did not follow patients beyond insulin initiation. Therefore, we do not know the extent to which our study subjects achieved glycemic control once therapy was intensified. We may also have underestimated the proportion of patients who initiated insulin because it could have been obtained from non-plan sources.
To maximize potential follow-up time, we limited the period in which patients could initiate SU/MET to 1996–2000. Under the HMO treatment algorithm then in place, most of our study subjects were receiving SU monotherapy before adding metformin. The current HMO treatment algorithm now recommends metformin as the first-line agent, followed by addition of SU. Our results may not apply to the new therapeutic pathway. Because we had relatively few subjects who added SU to metformin monotherapy, and even fewer who simultaneously initiated SU and metformin, we could not test whether different therapeutic pathways to SU/MET produced different levels of glycemic burden. However, a recent study showed that glycemic deterioration resumed in about 6 months after SU was added to metformin; the authors concluded that their results were consistent with UKPDS findings of early addition of metformin to SU monotherapy.4,7 Thus, it is unlikely that different pathways would generate different levels of glycemic burden.
Because diabetes is a progressive disease, many patients will eventually require insulin supplementation to achieve glycemic control targets.1 Patients in this setting treated with oral combination therapy are often not treated with maximal doses and appear to delay insulin initiation for prolonged periods, thereby, incurring substantial, and perhaps, preventable glycemic burden. These findings warrant additional studies examining the benefits of rapid titration to maximum doses and earlier initiation of insulin therapy. In addition, further research is needed to understand the clinician, patient, and system barriers to therapy intensification.
Acknowledgments
The authors wish to thank Jennifer Coury, for her editing suggestions, and James Hartnett, for his helpful review and comments. This work was supported by funding from Pfizer, Inc. and by the Kaiser Permanente Center for Health Research.
Conflict of Interest Statement Within the past 3 years, Gregory A. Nichols has received grant support from Pfizer, Inc.; GlaxoSmithKline, Inc; Merck & Co., Inc.; Eli Lilly and Co.; Sanofi-Aventis; and Bristol-Myers Squibb. Sonali N. Shah and Yuri Koo are employed by and hold stock in Pfizer, Inc.
Footnotes
Supported by a research grant from Pfizer, Inc.
Presented in poster form at the American Diabetes Association 66th Annual Meetings, June, 2006.
References
- 1.UKPDS Group. Glycemic control with diet, sulphonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999;281:2005–12. [DOI] [PubMed]
- 2.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. [DOI] [PubMed]
- 3.UKPDS Group. UKPDS 33: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with Type 2 diabetes. Lancet. 1998;352:837–51. [DOI] [PubMed]
- 4.UKPDS Group. UKPDS 34: Effect of intensive blood-glucose control with metformin on complications on overweight patients with type 2 diabetes. Lancet. 1998;352:854–65. [DOI] [PubMed]
- 5.Boccuzzi SJ, Wogen J, Fox J, Sung JCY, Shah AB, Kim J. Utilization of oral antihyperglycemic agents in a drug-insured US population. Diabetes Care. 2001;24:1411–15. [DOI] [PubMed]
- 6.Brown JB, Nichols GA. Slow response to loss of glycemic control in type 2 diabetes mellitus. Am J Manag Care. 2003;9:213–17. [PubMed]
- 7.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. [DOI] [PubMed]
- 8.De Witt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: a scientific review. JAMA. 2003;289:2254–64. [DOI] [PubMed]
- 9.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2006;29:1963–72. [DOI] [PubMed]
- 10.Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28:600–06. [DOI] [PubMed]
- 11.Grant RW, Cagliero E, Dubey AK, et al. Clinical inertia in the management of type 2 diabetes metabolic risk factors. Diabet Med. 2004;21:150–5. [DOI] [PubMed]
- 12.Grant RW, Buse JB, Meigs JB, The University HealthSystem Consortium (UHC) Diabetes Benchmarking Project Team. Quality of diabetes care in U.S. academic medical centers. Diabetes Care. 2005;28:337–42. [DOI] [PMC free article] [PubMed]
- 13.Polonsky WH, Fisher L, Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28:2543–5. [DOI] [PubMed]
- 14.Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673–9. [DOI] [PubMed]
- 15.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535–40. [DOI] [PubMed]
- 16.Hertz RP, Unger AN, Lustik MB. Adherence with pharmacotherapy for type 2 diabetes: a retrospective cohort study of adults with employer-sponsored health insurance. Clin Ther. 2005;27:1064–73. [DOI] [PubMed]
- 17.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. [DOI] [PubMed]
- 18.American Diabetes Association. Standards of medical care for patients with diabetes mellitus; clinical practice recommendations 2006. Diabetes Care. 2006;29:S1–S85. [DOI] [PubMed]
- 19.Stratton IM, Alder AI, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR, and on behalf of the UKPDS Study Group. UKPDS 35: association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 8-12-2000;21[7258], 405–12. [DOI] [PMC free article] [PubMed]
- 20.Leslie CA, Satin-Rapaport W, Matheson D, Stone R, Enfield G. Psychological insulin resistance: a missed diagnosis? Diabetes Spectr. 1994;7:52–7.
- 21.Rubin R, Peyrot M. Psychological issues and treatments in people with diabetes. J Clin Psychol. 2001;57:457–78. [DOI] [PubMed]
- 22.Koerbel G, Korytkowski M. Insulin therapy resistance: another form of insulin resistance in type 2 diabetes. Pract Diabetol. 2003;22:36–40.
- 23.Peyrot M. Psychological insulin resistance: overcoming barriers to insulin therapy. Pract Diabetol. 2004;23:6–12.
- 24.Polonsky WH, Jackson RA. What’s so tough about taking insulin? Addressing the problem of psychological insulin resistance in type 2 diabetes. Clin Diabetes. 2004;22:147–50. [DOI]
- 25.Nichols GA, Alexander CM, Girman CJ, Kamal-Bahl S, Brown JB. Treatment escalation and rise in HbA1c following successful initial Metformin therapy. Diabetes Care. 2006;29:504–9. [DOI] [PubMed]
- 26.Nichols GA, Alexander CM, Girman CJ, Kamal-Bahl S, Brown JB. A contemporary analysis of secondary failure of successful sulfonylurea therapy. Endocr Pract. 2006; (in press). [DOI] [PubMed]
- 27.Brown JB, Nichols GA, Glauber HS. Case-control study of 10 years of comprehensive diabetes care. West J Med. 2000;172:85–90. [DOI] [PMC free article] [PubMed]
- 28.Nichols GA, Glauber HS, Javor K, Brown JB. Achieving further glycemic control in type 2 diabetes mellitus. West J Med. 2000;173:5–9. [DOI] [PMC free article] [PubMed]
- 29.Willey CJ, Andrade SE, Cohen J, Fuller JC, Gurwitz JH. Polypharmacy with oral antidiabetic agents: an indicator of poor glycemic control. Am J Manag Care. 2006;12:435–440. [PubMed]


