Why Are the Nongenomic Effects of Estrogen So Important?
The work by Dr. Chaudry’s group in this issue of The American Journal of Pathology answers that question.1 We have long appreciated estrogen’s ability to modify a host’s response to a chronic process (genomic effects). However, the exciting prospect that estrogen may modify the acute response to injury through nongenomic pathways has only recently been appreciated (Figure 1). Indeed, the acute modification of the host’s response to injury by estrogen through the rapid signaling mechanisms of novel nongenomic pathways is clearly presented by the authors.1 From a clinical standpoint, this breakthrough is important. The ability to acutely change the outcome after severe injury has eluded investigators for at least a century. Understanding the signaling mechanisms that allow estrogen to accomplish rapid and dramatic effects will allow the development of refined therapeutics that may or may not require the broad ranging effects of a sex steroid such as estrogen. The important discoveries presented by Dr. Chaudry’s group in this issue of the AJP, and discussed in this commentary, confirm the notion that the discovery of the G-protein-coupled receptor (GPR)30-mediated estrogen effect considerably broadens the potential biological roles of estrogen.1 Through a series of complex mechanistic studies [involving both in vivo and in vitro work, small interferring RNA (siRNA) to suppress GPR30 and estrogen receptor (ER)-α expression, two forms of estrogen, and protein kinase A (PKA) inhibition], the authors demonstrate that estrogen rapidly protects hepatocytes when given after trauma and hemorrhage, during resuscitation. The combined use of bovine serum albumin-bound estrogen and GPR30 siRNA provides an elegant demonstration of the GPR30’s rapid effects while simultaneously differentiating its effects from those of the classic steroid hormone receptors in the membrane. They also demonstrate that the target kinase in this regard is PKA. In this single report, the authors 1) demonstrate that estrogen has rapid nongenomic effects; 2) show that these effects are biologically relevant in terms of the timing of a protective response after injury; 3) confirm the notion that nongenomic effects of estrogen considerably broaden its potential biological and therapeutic effects; 4) show that the cell surface receptor effect is mediated by GPR30 and not ER-α; and 5) clearly demonstrate the estrogen-to-GPR30-to-PKA protective link that may potentially yield the development of refined therapeutics.
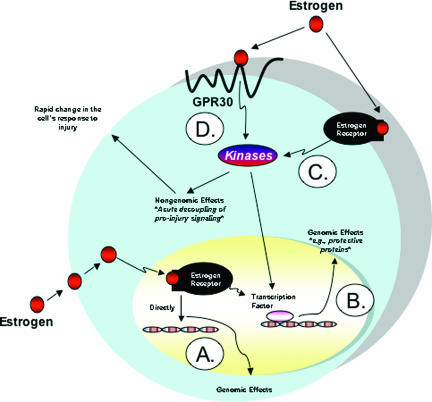
Figure 1.
Genomic and nongenomic effects of estrogen. A: The genomic effects of estrogen require that estrogen passively diffuse into the cell to act as a transcription factor after binding to its receptor. B: On the other hand, the complex may induce the production of a specific protein more indirectly through the activation of its transcription factor. These two mechanisms are now referred to as the genomic mechanisms of estrogen. The genomic mechanisms of estrogen rely on the production of proteins to mediate its effects, and consequently, such effects take longer to occur. In contrast, the nongenomic effects occur much more rapidly because they use existing proteins. C and D: Nongenomic effects may be mediated by classical-type estrogen receptors (C) residing in the cell membrane such as ER-α or nonclassical-type receptor proteins residing in the cell membrane such as the GPR30 (D). Modified from Figure 1 by Lorenzo.11
GPR30
Estrogens regulate a plethora of biological processes.1,2,3,4,5,6,7,8,9,10,11,12 Traditionally, it was held that all steroids, including estrogen, passively diffused into the cell2,3,11,12,13,14,15,16,17 to act as a transcription factor by binding to its receptor, which caused a change in its tertiary and quaternary structure to form the active complex. The active complex then bound the steroid response elements on the DNA upstream from steroid responsive genes, and transcription and translation of these genes resulted in proteins that ultimately were responsible for mediating estrogen’s effects (Figure 1A). Alternatively, the complex may induce the production of a specific protein15 more indirectly through the activation of its transcription factor (Figure 1B). We now refer to these two mechanisms as the genomic mechanisms of estrogen. The genomic mechanisms of estrogen rely on the production of proteins to mediate its effects, and consequently, such effects may be more gradual. In contrast, the nongenomic effects occur much more rapidly, taking only seconds, and use existing proteins for effect.2,3,13,14,18 These nongenomic effects may be mediated by classical-type estrogen receptors (Figure 1C) in the cell membrane (ie, ER-α) or nonclassical-type receptors in the cell membrane, such as the GPR30 (Figure 1D). In the article by Hsieh and colleagues,1 the authors report that estrogen’s protective effect was a cell surface-mediated, nongenomic effect mediated by a nonclassical estrogen receptor pathway. To discover this, they used an estrogen-protein complex that was able to activate cell membrane receptors but unable to diffuse into the nucleus to turn on the genomic effects. To differentiate whether the nongenomic effect was a classical estrogen receptor-mediated effect at the cell membrane or a nonclassical receptor-mediated effect, the authors used the two different forms of estrogen in combination with GPR30 siRNA and ER-α siRNA. This strategy revealed that GPR30 and not ER-α mediated the estrogen effect. Using the PKA inhibitor H89, they soundly established the estrogen-GPR30-PKA-BclII link, which is nicely shown in Figure 9 of their article.1
Clinical Studies Examining Gender Differences in Outcome Should Be Separated from the Potential Therapeutic Opportunities Offered by Estrogen’s Nongenomic Actions
We must not assume that nature has already done the study for us. For an eloquent argument on this subject as it relates to our generalized misinterpretation of estrogen in cardiovascular disease, please also see the discussion of Drs. Mendelsohn and Karas.19 To deny the potential therapeutic effects of estrogen based on any study that shows no gender differences in clinical outcomes may represent flawed logic. Controlled studies that involve the acute administration of a therapeutic dose of estrogen to elicit the nongenomic signaling pathways should first be accomplished. The study by Hsieh and colleagues1 have reaffirmed this notion. Indeed, we should endeavor to specifically evoke a nongenomic effect with a pulse of estrogen that is timed to the resuscitation period.
Nevertheless, some observational studies have shown a gender difference in outcomes after injury, even without controlling for hormone levels. Gender differences have been noted in outcome to acute injuries such as myocardial infarction, burns, trauma, and sepsis. Hospital-based clinical studies have shown that females have a higher mortality rate after myocardial infarction compared with males.16 In general, the females in many of these studies were older, had higher risk factors (diabetes, hypertension, and congestive heart failure), more complications, and lower likelihood of receiving treatment. Importantly, more males died from myocardial infarction before reaching the hospital, and the 28-day mortality for males and females was the same. This actually suggests that females are relatively protected in the immediate aftermath of a myocardial infarction but are similar to males at the end of a month.
Sepsis and trauma are two other inflammatory conditions associated with sex-dependent outcomes. For mortality in trauma, there is either no sex difference20,21,22 or sex difference in blunt trauma but not in penetrating trauma. Some studies showed benefit only in females >50 years old, whereas others showed benefit only in females <50 years old. Females, however, have lower incidence of pneumonia, sepsis, and multiorgan failure after trauma. In sepsis, some studies have found a higher mortality rate in females >80 years old, whereas others have found lower mortality rates for females. Among septic patients, females demonstrated lower mortality, higher interleukin-10, and lower tumor necrosis factor-α levels.10 Fewer female patients in intensive care units developed sepsis, although once sepsis developed, the mortality rate was the same. Clinical studies on sex differences in mortality after burns present inconsistent evidence. Some showed females or only females aged 30 to 59 years to have higher mortality, whereas others showed that females have lower incidence of multiorgan dysfunction and sepsis after burns.16
In contrast to the clinical studies, animal studies have consistently found that females do better. Protective effects of acute administration of estrogen, in an in vivo left anterior descending coronary artery ischemia/reperfusion model, have been shown in different animals. Chronic administration of estrogen provides protection from ischemia/reperfusion injury in isolated hearts undergoing global ischemia and in hearts undergoing in vivo left anterior descending obstruction. Estrogen also protected against reperfusion-induced arrhythmias after left anterior descending ischemia/reperfusion injury. Ovariectomized females have worse cardiac functional recovery after global ischemia/reperfusion than sham-ovariectomized females or ovariectomized females with estradiol replacement. After burn injury, females have lower cytokine production and better cardiac function. Trauma-hemorrhage leads to depressed immune function, which is more severe in males. The immune depression is in part caused by testosterone because both castration and receptor blockade attenuated this depression. Estrogen also prevented the immune depression caused by trauma-hemorrhage.16
Animal studies have consistently shown that females are protected against acute injury, whereas clinical studies seem inconsistent. A possible reason is that in animal studies, the female population is well controlled and only proestrous females are used, whereas clinical studies have a heterogeneous population. Furthermore, the underlying condition of humans is less uniform. Indeed, the few animal studies that used diestrous females showed that diestrous females had functional recovery equivalent to males, but lower than proestrous females. This has been borne out by a few clinical studies that showed that cardiac function fluctuates with the hormonal changes of the menstrual cycle.16 Thus, it is important to know the hormonal status of females and future clinical studies that take this into account may produce more consistent results. More importantly, given the data presented by Drs. Hsieh and Chaudry1 in this issue of the AJP, clinical studies should focus on acute activation of the nongenomic estrogen-signaling pathways through GPR30.
Estrogen in Acute Resuscitation Scenarios: Historical Aspects
Dr. Chaudry and colleagues23 first discovered that females were more resistant to experimental sepsis when compared with males. Subsequently, they were also the first to note that males fared worse after trauma-hemorrhage when compared with females.24,25 Most great discoveries were made by chance, and my personal communication with the authors revealed that this was no exception. Before 1995, when this discovery was made, nearly all shock and trauma research was performed in male animals. Not suspecting that it mattered, one of Dr. Chaudry’s postdoctoral fellows, Dr. Rene Zellweger, used a batch of female animals for his experiments, instead of males.23 He observed that the female animals were not as susceptible to Dr. Chaudry’s cecal ligation and puncture26 method of inducing intra-abdominal sepsis. Although this was probably somewhat disappointing to the postdoctoral fellow initially (who would have thought that gender would make such a difference in the outcome to an acute process such as cecal ligation and puncture?), his mentor, Dr. Chaudry, asked his post-doctoral fellow to pursue the unexpected findings with vigor. In retrospect, this insight is remarkable because in 1995 the shock and trauma community was not entertaining such ideas. The community was just becoming accustomed to the bias that females may be more protected from disease processes that followed the model of: injury → chronic inflammation → repair, such as atherosclerosis. However, because it was widely held that estrogen operated through genomic mechanisms, its effect on acute injury, inflammation, and repair had not yet been examined in the shock models. The work accomplished since that original observation brings us to this issue’s important discovery,1 which I believe will prove to be every bit as significant. The article by Dr. Irshad Chaudry and colleagues in this issue of the AJP1 should be viewed within the broader context of his life’s work in shock research that includes the basic inflammatory responses to sepsis, ischemia and reperfusion injury, burn, and hypovolemic shock, with and without concomitant tissue trauma. Indeed, Dr. Chaudry introduced and established every clinically relevant contemporary shock model. He has characterized the organ injury mechanisms and inflammatory responses in each of these models, and Dr. Chaudry’s concepts and mechanisms of shock-induced injury are the premise from which most shock investigators work. He has presented a multitude of potent therapies throughout the years and it is fascinating to posit that perhaps the most potent therapy may reside in the novel nonclassical, nongenomic signaling mechanisms of estrogen such as GPR30, originally discovered by a combination of chance, keen observational skills, and a seasoned investigator’s instinct.
Footnotes
Address reprint requests to Daniel R. Meldrum, 545 Barnhill Dr., Emerson Hall 215, Indianapolis, IN 46202. E-mail: dmeldrum@iupui.edu.
Related article on page 1210
This commentary relates to Hsieh et al, Am J Pathol 2007, 170:1210–1218, published in this issue.
References
- Hsieh YC, Yu HP, Frink M, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury following trauma-hemorrhage. Am J Pathol. 2007;170:1210–1218. doi: 10.2353/ajpath.2007.060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1). J Mol Cell Cardiol. 2007;42:142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisostomo PR, Wang M, Herring CM, Morrell ED, Seshadri P, Meldrum KK, Meldrum DR. Sex dimorphisms in activated mesenchymal stem cell function. Shock. 2006;26:571–574. doi: 10.1097/01.shk.0000233195.63859.ef. [DOI] [PubMed] [Google Scholar]
- George RL, McGwin G, Jr, Schwacha MG, Metzger J, Cross JM, Chaudry IH, Rue LW., III The association between sex and mortality among burn patients as modified by age. J Burn Care Rehabil. 2005;26:416–421. doi: 10.1097/01.bcr.0000176888.44949.87. [DOI] [PubMed] [Google Scholar]
- Hildebrand F, Hubbard WJ, Choudhry MA, Frink M, Pape HC, Kunkel SL, Chaudry IH. Kupffer cells and their mediators: the culprits in producing distant organ damage after trauma-hemorrhage. Am J Pathol. 2006;169:784–794. doi: 10.2353/ajpath.2006.060010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YC, Choudhry MA, Yu HP, Shimizu T, Yang S, Suzuki T, Chen J, Bland KI, Chaudry IH. Inhibition of cardiac PGC-1alpha expression abolishes ERbeta agonist-mediated cardioprotection following trauma-hemorrhage. FASEB J. 2006;20:1109–1117. doi: 10.1096/fj.05-5549com. [DOI] [PubMed] [Google Scholar]
- Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, III, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- Jarrar D, Kuebler JF, Wang P, Bland KI, Chaudry IH. DHEA: a novel adjunct for the treatment of male trauma patients. Trends Mol Med. 2001;7:81–85. doi: 10.1016/s1471-4914(01)01917-7. [DOI] [PubMed] [Google Scholar]
- Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res. 2005;67:594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- Lorenzo J. A new hypothesis for how sex steroid hormones regulate bone mass. J Clin Invest. 2003;111:1641–1643. doi: 10.1172/JCI18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol. 2006;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6:127–135. doi: 10.2174/187153006777442422. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24(Suppl 1):101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- Meldrum DR. Estrogen increases protective proteins following trauma and hemorrhage. Am J Physiol. 2006;290:R809–R811. doi: 10.1152/ajpregu.00802.2005. [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Wang M, Tsai BM, Kher A, Pitcher JM, Brown JW, Meldrum KK. Intracellular signaling mechanisms of sex hormones in acute myocardial inflammation and injury. Front Biosci. 2005;10:1835–1867. doi: 10.2741/1665. [DOI] [PubMed] [Google Scholar]
- Schneider CP, Schwacha MG, Chaudry IH. Impact of gender and age on bone marrow immune responses in a murine model of trauma-hemorrhage. J Appl Physiol. 2007;102:113–121. doi: 10.1152/japplphysiol.00848.2006. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Samy TS, Schwacha MG, Wang P, Rue LW, III, Bland KI. Endocrine targets in experimental shock. J Trauma. 2003;54:S118–S125. doi: 10.1097/01.TA.0000064511.14322.F1. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- Napolitano LM, Greco ME, Rodriguez A, Kufera JA, West RS, Scalea TM. Gender differences in adverse outcomes after blunt trauma. J Trauma. 2001;50:274–280. doi: 10.1097/00005373-200102000-00013. [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma. 2000;48:932–937. doi: 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- Gannon CJ, Napolitano LM, Pasquale M, Tracy JK, McCarter RJ. A statewide population-based study of gender differences in trauma: validation of a prior single-institution study. J Am Coll Surg. 2002;195:11–18. doi: 10.1016/s1072-7515(02)01187-0. [DOI] [PubMed] [Google Scholar]
- Zellweger R, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state tolerate sepsis better than males. Surg Forum. 1995;46:65–67. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Enhanced immune responses in females, as opposed to decreased responses in males following haemorrhagic shock and resuscitation. Cytokine. 1996;8:853–863. doi: 10.1006/cyto.1996.0114. [DOI] [PubMed] [Google Scholar]
- Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106–110. doi: 10.1097/00003246-199701000-00021. [DOI] [PubMed] [Google Scholar]
- Chaudry IH, Wichterman KA, Baue AE. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979;85:205–211. [PubMed] [Google Scholar]