Abstract
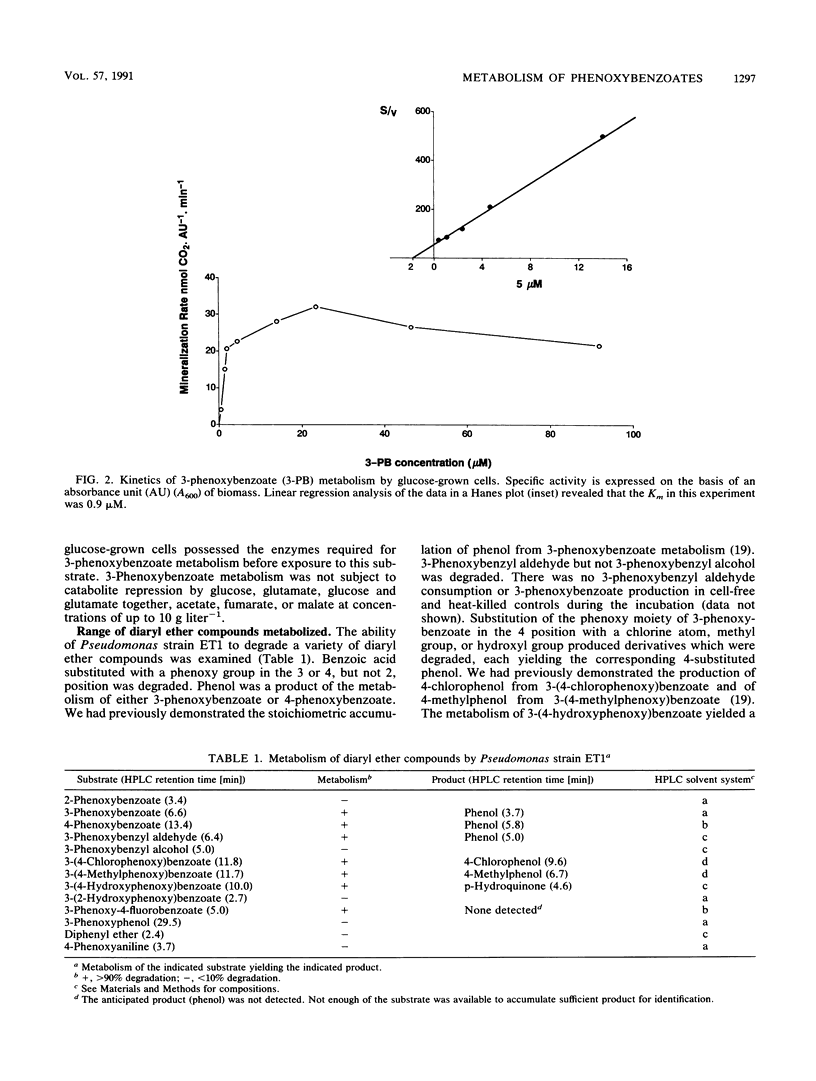
3-Phenoxybenzoate is a transient metabolite from the breakdown of a number of pyrethroid insecticides in soil. In this study, we identified and characterized a bacterium which could grow on 3-phenoxybenzoate, converting it to phenol. On the basis of morphological and biochemical features, the 3-phenoxybenzoatedegrading isolate was determined to be a Pseudomonas species, probably a strain of Pseudomonas delafieldii, now designated Pseudomonas strain ET1. Pseudomonas strain ET1 grew on 3-phenoxybenzoate with a generation time of 3 h and a specific rate of metabolism of (2.6 ± 0.9) × 10-13 g of 3-phenoxybenzoate consumed cell-1 h-1. The Km for 3-phenoxybenzoate metabolism was 1.4 ± 0.8 μM. The metabolism of 3-phenoxybenzoate was constitutive and not subject to catabolite repression. The metabolism of a variety of substituted diaryl ether compounds was examined. 3- and 4-Phenoxybenzoates were metabolized, but 2-phenoxybenzoate was not. Phenoxy-substituted benzyl aldehyde was metabolized, but phenoxy-substituted benzyl alcohol, benzene, phenol, and aniline were not. Derivatives of 3-phenoxybenzoate substituted in the 4′ position with hydroxyl, methyl, or chlorine were metabolized, yielding the corresponding 4-substituted phenol. 3-(2-Hydroxyphenoxy)benzoate was not metabolized, but 3-phenoxy-4-fluorobenzoate was. These results indicate that the metabolism of the tested diaryl ether compounds was restricted to 4-phenoxybenzoate, 3-phenoxybenzyl aldehyde, and 3-phenoxybenzoate derivatives without a substitution in the 2′ position.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis D. H., Stanier R. Y., Doudoroff M., Mandel M. Taxonomic studies on some gram negative polarly flagellated "hydrogen bacteria" and related species. Arch Mikrobiol. 1970;70(1):1–13. doi: 10.1007/BF00691056. [DOI] [PubMed] [Google Scholar]
- Engesser K. H., Fietz W., Fischer P., Schulte P., Knackmuss H. J. Dioxygenolytic cleavage of aryl ether bonds: 1,2-dihydro-1,2-dihydroxy-4-carboxybenzophenone as evidence for initial 1,2-dioxygenation in 3- and 4-carboxy biphenyl ether degradation. FEMS Microbiol Lett. 1990 Jun 1;57(3):317–321. doi: 10.1016/0378-1097(90)90087-7. [DOI] [PubMed] [Google Scholar]
- Lynch M. J., Wopat A. E., O'connor M. L. Characterization of two new facultative methanotrophs. Appl Environ Microbiol. 1980 Aug;40(2):400–407. doi: 10.1128/aem.40.2.400-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney S. E., Maule A., Smith A. R. Microbial transformation of the pyrethroid insecticides: permethrin, deltamethrin, fastac, fenvalerate, and fluvalinate. Appl Environ Microbiol. 1988 Nov;54(11):2874–2876. doi: 10.1128/aem.54.11.2874-2876.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. K., Scow K. M., Alexander M. Kinetics of p-nitrophenol mineralization by a Pseudomonas sp.: effects of second substrates. Appl Environ Microbiol. 1987 Nov;53(11):2617–2623. doi: 10.1128/aem.53.11.2617-2623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Topp E., Crawford R. L., Hanson R. S. Influence of readily metabolizable carbon on pentachlorophenol metabolism by a pentachlorophenol-degrading Flavobacterium sp. Appl Environ Microbiol. 1988 Oct;54(10):2452–2459. doi: 10.1128/aem.54.10.2452-2459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitzur S. Rapid determnation of DNA base composition by ultraviolet spectroscopy. Biochim Biophys Acta. 1972 Jun 22;272(1):1–11. doi: 10.1016/0005-2787(72)90025-1. [DOI] [PubMed] [Google Scholar]
- Unai T., Casida J. E. Synthesis of isomeric 3-(2,2-dichlorovinyl)-2-hydroxymethyl-2-methylcyclopropanecarboxylic acids and other permethrin metabolites. J Agric Food Chem. 1977 Sep-Oct;25(5):979–987. doi: 10.1021/jf60213a006. [DOI] [PubMed] [Google Scholar]



