Abstract
The precise role of insulin-like growth factor (IGF)-1 in gastric ulcer healing is unknown. In experimental rat gastric ulcers, we examined expression of IGF-1 mRNA and protein by reverse transcriptase-polymerase chain reaction or enzyme-linked immunosorbent assay and immunostaining, respectively. In cultured rat gastric epithelial RGM1 cells, we examined effects of exogenous IGF-1 on cell migration, re-epithelialization, and proliferation—essential components of ulcer healing. We also examined whether IGF-1 induces cyclooxygenase (COX)-2 expression and determined the role of phosphatidylinositol 3-kinase and mitogen-activated protein kinase signaling pathways in mediating IGF-1 actions. Gastric ulceration triggered an approximately threefold increase in IGF-1 expression in epithelial cells of the ulcer margins (P < 0.001 versus control), especially in cells re-epithelizing granulation tissue and in mucosa in proximity to the ulcer margin. Treatment of RGM1 cells with IGF-1 caused a dramatic increase in actin polymerization, an eightfold increase in cell migration (P < 0.001), a 195% increase in cell proliferation (P < 0.05), and a sixfold increase in COX-2 expression (P < 0.01). Inhibitor of phosphatidylinositol 3-kinase abolished IGF-1-induced RGM1 cell migration and proliferation, actin polymerization, and COX-2 expression. The up-regulation of IGF-1 in gastric ulcer margin accelerates gastric ulcer healing by promoting cell re-epithelization, proliferation, and COX-2 expression via the phosphatidylinositol 3-kinase pathway.
Gastric ulcer healing is a complex process involving inflammation, re-epithelialization, formation of granulation tissue, angiogenesis, interactions between various cells and matrix, and tissue remodeling.1,2 Growth factors such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF) activate epithelial cell migration and proliferation and accelerate ulcer healing in vivo and in vitro by interacting with specific cell surface receptors, which initiate cascades of intracellular events.1,2,3
Insulin-like growth factor-1 (IGF-1) is a peptide that binds to IGF receptor-1 (IGFR-1), a tyrosine kinase membrane receptor. Activation of IGFR-1 by IGF-1 is implicated in cell survival, growth, differentiation, and migration in epithelial and mesenchymal tissues.4,5 In the gastrointestinal tract, IGF-1 is secreted by salivary and other exocrine glands, and its receptor is present in epithelial cells of all segments of the rat gastrointestinal tract.6,7,8 In addition, IGF-1 has recently been shown to stimulate intrahepatic biliary epithelial cell proliferation.9 Several studies have demonstrated IGF-1 up-regulation in injured skin, bone, and brain.10,11,12 Whether gastrointestinal tract ulceration affects IGF-1 expression is unknown. Previous studies in diabetic and arthritis rat models have demonstrated a delay in gastric ulcer healing and attributed it to a decrease in IGF-1 mRNA in the gastric mucosa.13,14 Injection of exogenous IGF-1 to these diabetic and arthritic rats accelerated ulcer healing. Direct injection of IGF-1 into the ulcers was also shown to accelerate healing of cryo-induced rat gastric ulcers.15 Under in vitro condition, exogenous IGF-1 has been shown to promote migration and proliferation in wounded monolayer of rabbit gastric epithelial cells,16,17 but the molecular mechanisms and signaling pathways of these actions remain unexplained.
The aim of this study was to determine in the in vivo rat gastric ulcer model the effect of gastric ulceration on expression and localization of IGF-1. In cultured rat gastric mucosal epithelial RGM1 cells, we examined whether and how IGF-1 promotes gastric epithelial cell migration and proliferation and studied the effect of IGF-1 on cyclooxygenase (COX)-2 expression. In the same in vitro model, we examined signaling pathways mediating these actions of IGF-1.
Materials and Methods
Rat Gastric Ulcer Induction
This study was approved by the Subcommittee for Animal Studies of Veterans Administration Long Beach Health Care System. Male Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA) weighing 225 to 250 g were fasted for 16 hours before surgery. The rats were anesthetized with 50 mg/kg pentobarbital by intraperitoneal injection. Gastric ulcers were induced in rats by a focal, serosal application of 100% acetic acid to the glandular portion of the stomach for 90 seconds by using a 4.0-mm inner diameter polyethylene tube as previously described.18 A separate group of rats was subjected to sham operation without application of acetic acid. Rats were euthanized at 4, 6, and 12 days after ulcer induction, the stomach opened, and mucosal specimens of ulcerated or control mucosa obtained. Specimens were used for RNA extraction and protein isolation or fixed in 10% formalin for immunohistochemistry.
Enzyme-Linked Immunosorbent Assay
Total protein from control and ulcerated mucosa was isolated by homogenization of tissues in ice-cold radioimmunoprecipitation assay buffer containing protease inhibitor cocktail (Pierce, Rockford, IL). Protein concentration was determined by standard Bradford assay (Bio-Rad, Hercules, CA), and samples were used to assay for rat IGF-1. We used a commercially available mouse IGF-1 enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN), which detects rat IGF-1, and followed the manufacturer’s protocol for assays. Colorimetric measurements were performed using a microplate reader (Dynex Technologies, San Francisco, CA) at 490 nm. Measurements were done on triplicate samples and repeated twice.
Immunohistochemistry
Immunostaining was performed as previously described.18 Specimens of control and ulcerated stomach were fixed in 10% formalin, routinely processed, paraffin-embedded, and sectioned. Sections were dewaxed and processed routinely using DAKO Target retrieval solution (DAKO, Carpinteria, CA) for unmasking of antigen before blocking. Primary antibody used was a rabbit polyclonal IGF-1, 1:200 dilution (catalog no. 9013; Santa Cruz Biotechnology, Santa Cruz, CA). After incubation with primary antibodies, slides were then treated with appropriate secondary antibody for 60 minutes and developed using the ABC detection method.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA from normal control mucosa, ulcer margins, and RMG1 cells was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Total RNA was used as template for first-strand cDNA synthesis with reverse transcriptase (Perkin Elmer, Norwalk, CT). The cDNA was then used as templates for PCR reactions as previously described by our laboratory19 using the following set of primers: IGF-1 forward 5′-CAGTTCGTGTGTGGACCAAG-3′ and IGF-1 reverse 5′-GTCTTGGGCATGTCAGTGTG-3′. The sequences for rat COX-2 were previously described19; β-actin primers were purchased from Clontech Laboratories, Inc., Palo Alto, CA, and used as internal control.
Cell Culture and Cell Migration Studies
Rat gastric mucosal epithelial (RGM1) cell line derived from normal rat gastric mucosa was obtained from Riken Cell Bank (Tsukuba, Japan).20 Cells were maintained in Dulbecco’s modified Eagle’s medium/F12 medium supplemented with 2 mmol/L l-glutamine, antibiotics/anti-mycotics, and 20% fetal bovine serum at 37°C with 5% CO2 in a humidified incubator. To determine the effects of IGF-1 on RGM1 cell migration, we performed scratch-wound assay as described in previous studies.21,22 In brief, RGM1 cells were plated on a six-well plate at a density of ∼1 × 105 cells per well and incubated with growth medium for 3 days until fully confluent. Cells were rinsed three times in phosphate-buffered saline (PBS) before being subjected to wound/injury by scraping the monolayer with a single edge razor blade across the well. The cells were rinsed in PBS and incubated in serum-free medium without or with rat IGF-1 (Cell Sciences, Canton, MA) at 5, 10, or 50 ng/ml. After 24 hours, cells were fixed in ice-cold methanol (−20°C) for 5 minutes and stained with hematoxylin and eosin. Cell migration was determined by counting the number of RGM1 cells migrating across the cut border for 24 hours. To determine the signaling pathways by which IGF-1 affects RGM1 cell migration, cells were placed in serum-free medium overnight and then preincubated with vehicle, the phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 (Calbiochem, San Diego, CA) at 20 μmol/L, or the MAPK (MEK-1/2) inhibitor PD98059 (Calbiochem) at 40 μmol/L for 30 minutes followed by treatment with IGF-1 (10 ng/ml) for 24 hours. The doses of these inhibitors (PD98059 and LY294002) were selected based on previous dose-response studies.23,24
To determine the role of COX-2 in IGF-1-induced RGM1 cell migration, cells were preincubated with COX-2 selective inhibitor, NS-398 (Cayman Chemical Company, Ann Arbor, MI) at 50 μmol/L for 2 hours before treatment with IGF-1 (10 ng/ml) for 24 hours. We selected the dose of NS-398 based on previous studies investigating kinetics of purified human COX-1 and COX-2 inhibition, which demonstrated that NS-398 inhibits human COX-1 and human COX-2 with a selectivity of ∼260-fold for COX-2 over COX-1 and that in this concentration NS-398 (50 μmol/L) does not inhibit COX-1.25,26 To determine whether IGF-1 plus EGF is more effective (compared with either growth factor alone) in inducing cell migration, serum-starved wounded monolayer cells were incubated with either IGF-1 (10 ng/ml) alone or EGF (10 ng/ml) alone, or with IGF-1 plus EGF for 24 hours. To determine the relative contribution of cell proliferation to RGM1 cell migration, cell proliferation was inhibited by preincubating with 2 μg/ml mitomycin C for 2 hours before incubation with IGF-1 as previously described.22 All cell migration studies were done in triplicates and repeated twice; three random microscopic fields were selected for counting in each well, and average values were attained (n = 9).
Cell Proliferation Assay
The effect of IGF-1 on cell proliferation was studied by determining [3H]thymidine incorporation into cell DNA as described in our previous study.27 RGM1 cells were seeded at ∼2 × 104 cells per well in a 24-well tissue culture dish and were grown overnight to ∼60% confluence. The cells were then placed for 24 hours in serum-free medium and treated with 0, 5, 10, or 50 ng/ml IGF-1 for another 24 hours. Three hours before the termination of the experiment, 2 μCi of [3H]thymidine (Amersham Pharmacia Biotech, Piscataway, NJ) was added to each well. To determine the signaling pathways by which IGF-1 affects RGM1 cell proliferation, cells were placed in serum-free medium overnight and then preincubated with either vehicle, the PI3K inhibitor LY294002 at 20 μmol/L, or the MAPK (MEK-1/2) inhibitor PD98059 at 40 μmol/L for 30 minutes before IGF-1 treatment. To determine the role of COX-2 in IGF-1 action, cells were incubated with NS-398 (50 μmol/L) for 2 hours before the addition of IGF-1. After 24 hours, cells were washed three times in ice-cold PBS and lysed in 0.5 N NaOH, and radioactivity was measured in a scintillation counter. All cell proliferation studies were done in triplicates and repeated twice (n = 9). In addition, we used bromodeoxyuridine (BrdU) labeling to assure that changes in [3H]thymidine uptake were not attributable to changes in TdR kinase activity. For the BrdU labeling, cells were plated on 12-well coverslips coated with rat collagen gel. Cells were treated with IGF-1 at 1, 10, and 50 ng/ml or medium alone (control) for 24 hours and were incubated with BrdU at final concentration at 10 μmol/L for 2 hours before termination of the experiment. Each study was performed in triplicate. After fixation and treatment with 2 N HCl at 37°C for 1 hour, cells were incubated with anti-BrdU antibody (Chemicon, Temecula, CA), rinsed with PBS, incubated with Alexa 488-conjugated secondary antibody, and evaluated under a Nikon fluorescence microscope (Tokyo, Japan). BrdU-positive cells were counted in five randomly selected areas in each coverslip.
Actin Polymerization—Labeling of G-Actin and F-Actin
The effect of exogenous rat IGF-1 on actin polymerization was examined by staining RGM1 cells with fluorescent probes for G- and F-actin as described previously.28 Cells were plated on collagen-coated coverslips and grown to fully confluent before excision wounding. Cells were then washed in PBS and incubated in serum-free medium for 24 hours, then treated with IGF-1 (10 ng/ml) for 24 hours, with or without LY294002 preincubation, for 30 minutes. Cells were then fixed in 4% paraformaldehyde, permeabilized in cold (−20°C) acetone, and double-stained for 30 minutes with Texas Red-conjugated deoxyribonuclease I (Molecular Probes, Eugene, OR), which binds to monomeric, nonpolymerized G-actin, and Oregon Green 488-conjugated phallotoxins (Molecular Probes), which bind to polymerized F-actin. The staining was visualized under a Nikon fluorescence microscope as previously described.28
Protein Extraction and Immunoblotting
After completion of experiments, RGM1 cells were rinsed in cold PBS, harvested in ice-cold radioimmunoprecipitation assay buffer, and processed as previously described. Protein concentration was determined by standard Bradford assay (Bio-Rad). For immunoblotting, equal amounts of protein were loaded into a standard 10% sodium dodecyl sulfate-polyacrylamide gel and resolved overnight. The resolved proteins were transferred to a nitrocellulose membrane by wet transfer method for 2 hours; membranes were blocked in blocking buffer (1× Tris-buffered saline, 0.1% Tween 20, and 5% nonfat dry milk) before incubation with primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. Detection of protein was performed with chemiluminescence reagents (Amersham Pharmacia Biotech) as previously described.28
COX-2 Expression in RGM1 Cells and Signaling Pathways Involved
RGM1 cells were plated at ∼1 × 106 cells per plate in a 100-mm dish and incubated in growth medium overnight to ∼70% confluence. Cells were rinsed in PBS and incubated in Dulbecco’s modified Eagle’s medium/F12 (serum-free) medium only for 24 hours. For the dose-dependent studies, cells were placed in above medium or treated for 6 and 24 hours with IGF-1 at 10, 50, or 100 ng/ml or with 100 ng/ml EGF. Extracted proteins were immunoblotted with COX-2-specific polyclonal antibody (Cayman Chemical Company). For time-dependent studies, cells were treated with 10 ng/ml IGF-1. Cells were harvested after 10, 30, 60, 180, or 360 minutes of stimulation, and isolated proteins were immunoblotted with COX-2-specific polyclonal antibody, phospho-Akt (Cell Signaling Technology, Beverly, MA), total-Akt (Santa Cruz Biotechnology) or phospho-extracellular signal-regulated kinase (ERK) 1/2 (Santa Cruz Biotechnology), total ERK2 (Santa Cruz Biotechnology), and β-actin (Sigma-Aldrich, St. Louis, MO).
Statistical Analysis
All numeric data are expressed as mean ± SD. Student’s two-tailed t-test was used to compare data between two groups. One-way analysis of variance with Bonferroni post hoc validation was used to compare data among three or more groups. P < 0.05 was considered significant.
Results
Gastric Ulceration Triggers Local Activation of IGF-1 in the Ulcer Margins
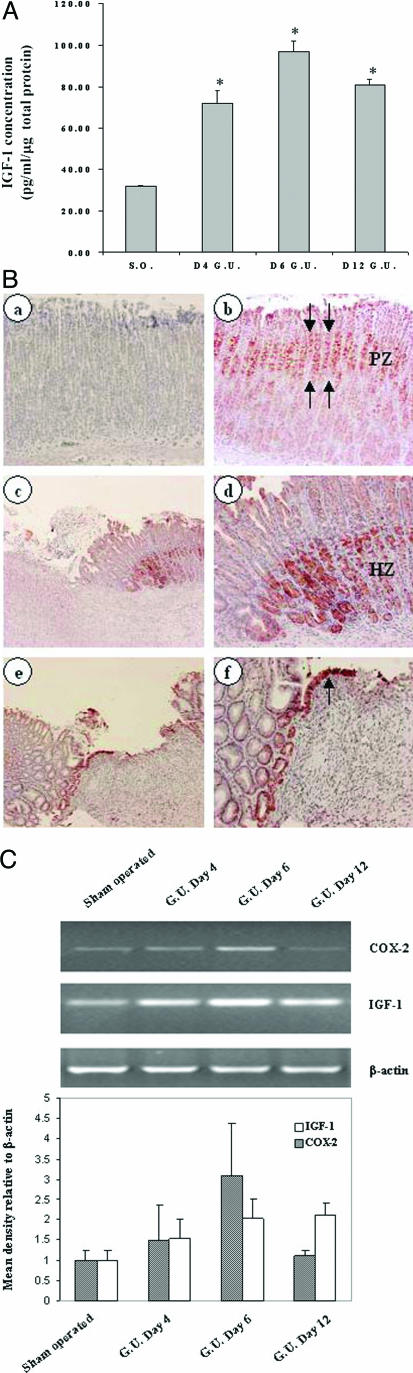
To measure IGF-1 concentration in control and ulcerated gastric tissues, we performed enzyme-linked immunosorbent assay using total protein extract prepared from gastric mucosa of sham-operated and gastric ulcer margins obtained 4, 6, and 12 days after ulcer induction. IGF-1 concentration in ulcerated gastric mucosa was significantly increased by 227, 305, and 255% at 4, 6, and 12 days, respectively, versus that of sham-operated rats. The absolute IGF-1 concentrations were 71.9 ± 6, 97.0 ± 5, and 80.7 ± 3 pg/ml/μg total protein versus 31.7 ± 0.5 pg/ml/μg total protein, respectively (Figure 1A).
Figure 1.
Gastric ulceration triggers local activation of IGF-1 in the ulcer margins. A: IGF-1 enzyme-linked immunosorbent assay of gastric tissue from sham-operated (S.O.) and gastric ulcers showing significant IGF-1 up-regulation at days 4, 6, and 12 after gastric ulcer (G.U.) induction. *P value <0.001, n = 4 to 5. B: Immunostaining with IGF-1-specific antibody. In normal gastric mucosa, IGF-1 expression is localized to cells of mucosal progenitor zone, PZ (b) (red staining). In ulcerated mucosa, IGF-1 expression is up-regulated in gastric ulcer margin healing zone, HZ, at day 6 (c and d) compared with normal gastric mucosa. d: Higher magnification of the same section showing IGF-1-positive cells are derived from gastric glands. e and f: Epithelial cells migrating onto and re-epithelizing granulation tissue of gastric ulcers at day 6 also exhibit strong staining for IGF-1 (arrow). a: Control staining performed in the absence of primary antibody did not show any positive staining. C: RT-PCR using Cox-2, Igf-1, and β-actin primers was performed using total RNA from gastric tissue of sham-operated control and gastric ulcer margins showing Igf-1 and Cox-2 mRNA up-regulation at days 4, 6, and 12. Semiquantification of RNA RT-PCR expressed as relative density to β-actin (bottom). Original magnifications: ×200 (Bd, Bf); ×100 (Be).
To assess expression and localization of IGF-1 in rat gastric ulcers, we stained sections from normal and ulcerated gastric mucosa with a specific antibody against IGF-1. In normal rat gastric mucosa, IGF-1 was predominantly expressed in the progenitor (proliferative zone) cells (Figure 1Bb). In rat gastric ulcer at day 6, IGF-1 expression was localized to epithelial cells that are in proximity to the base of the ulcer margin (Figure 1B, c and d). A strong IGF-1 staining was also present in the epithelial cells that migrated onto granulation tissue during re-epithelization phase of gastric ulcer healing (Figure 1B, e and f). To determine IGF-1 mRNA expression during gastric ulcer healing, we performed semiquantitative RT-PCR using RNA prepared from gastric ulcer margins at 4, 6, and 12 days after ulcer induction. IGF-1 mRNA in rat gastric ulcer margins was markedly increased at 4, 6, and 12 days after ulcer induction versus sham-operated rats. COX-2 mRNA expression was also up-regulated in gastric ulcer margin, with a peak at day 6 and returned to near baseline level by day 12 (Figure 1C).
IGF-1 Induces RGM1 Cell Migration and Proliferation
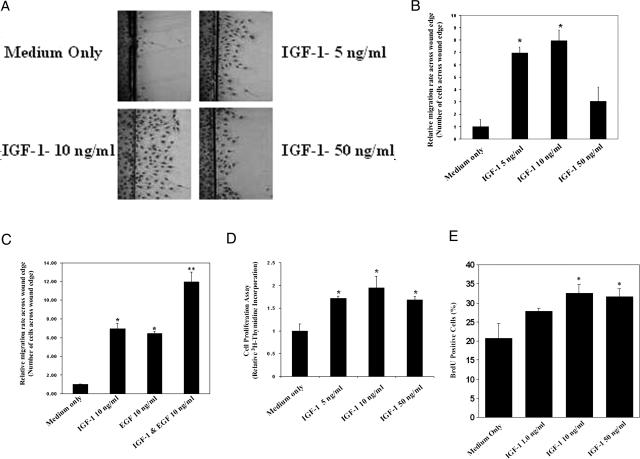
One of the major steps in ulcer healing is re-epithelialization, which is usually achieved by epithelial cell migration and proliferation.1,2,3 To determine the role of IGF-1 in cell migration, we examined RGM1 cell migration in scratch wound assay across wound edge after 24-hour treatment with IGF-1 or medium only control. Treatment with IGF-1 at 5, 10, and 50 ng/ml significantly increased the RGM1 cell migration rate by sevenfold, eightfold, and threefold, respectively, compared with controls (Figure 2, A and B). The addition of both IGF-1 (10 ng/ml) plus EGF (10 ng/ml) to culture medium had an additive effect and significantly increased cell migration by approximately twofold versus either single growth factor alone (Figure 2C). Because the concentration of 10 ng/ml IGF-1 induced maximal migration, all subsequent migration studies were done with 10 ng/ml IGF-1. This concentration is considered within physiological range because serum levels of IGF-1 in humans are ∼120 ng/ml.29
Figure 2.
IGF-1 induces RGM1 cell migration and proliferation in vitro. IGF-1 plus EGF exert additive effect on RGM1 cell migration. A: A representative photomicrograph of RGM1 cells migrating across wound edge. There is increased cell migration in response to treatment with 5, 10, and 50 ng/ml IGF-1 compared with medium only control. B: Data for cell migration studies showing a peak cell migration in the presence of 10 ng/ml IGF-1. *P value <0.001 compared with medium only control. All cell migration studies were done in triplicates and repeated twice; three random microscopic fields were selected for counting in each experiment (n = 9). C: Treatment with IGF-1 plus EGF exerts an additive effect on cell migration compared with either growth factor alone. *P value <0.05 compared with medium only control. **P value <0.05 compared with IGF-1 alone or EGF alone. D: Proliferation assay showing an increase in [3H]thymidine incorporation in RGM1 cells treated with 5, 10, and 50 ng/ml IGF-1 for 24 hours compared with medium only control. Experiments were done in triplicates and repeated twice (n = 9). E: Treatment with IGF-1 significantly increases BrdU uptake by RGM1 cells. *P value <0.05 compared with medium only control.
We then examined the effect of IGF-1 on RGM1 cell proliferation using [3H]thymidine incorporation assays. Treatment with 5, 10, or 50 ng/ml IGF-1 increased cell proliferation by 1.7-, 2.0-, and 1.7-fold, respectively, compared with medium only control (P < 0.05) (Figure 2D). The BrdU labeling assay fully confirmed these data. Treatment with IGF-1 at 1, 10, and 50 ng/ml increased RGM1 cell proliferation by 35%, ∼60% (P < 0.05), and 58% (P < 0.05), respectively (Figure 2E).
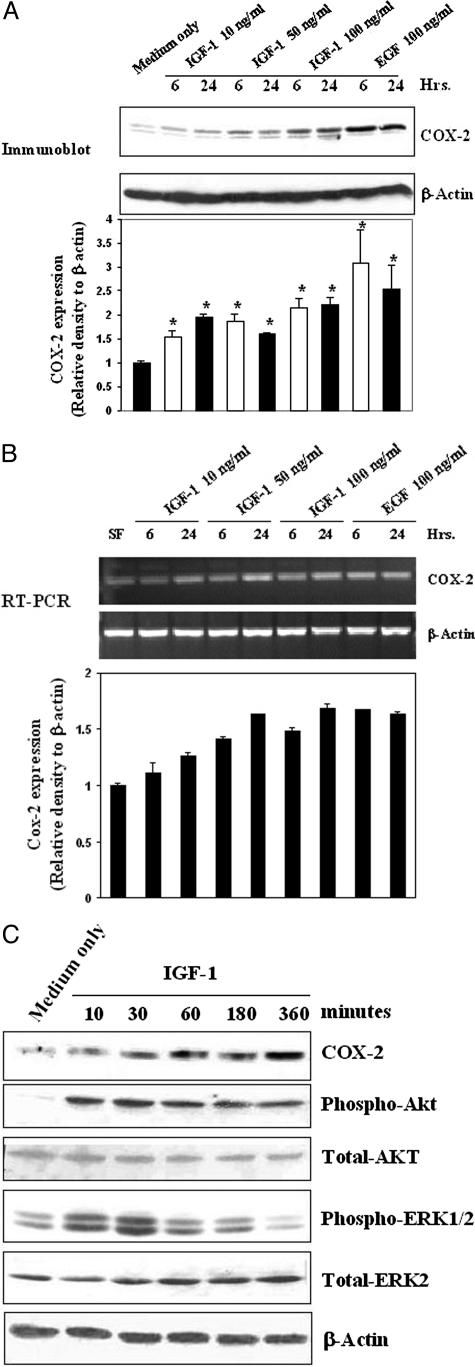
IGF-1 Activates COX-2 Expression in RGM1 Cells
It has been shown that COX-2 can be induced in vitro in gastric epithelial cells by several growth factors and that it is also up-regulated in the gastric ulcer margin.19,30,31,32,33 We examined whether IGF-1 can induce COX-2 expression and used EGF, which is known to induce COX-2 expression, as a positive control. IGF-1 treatment for 6 and 24 hours significantly increased COX-2 expression (Figure 3A). EGF (100 ng/ml) also significantly increased COX-2 expression compared with medium only (Figure 3A). The semiquantitative RT-PCR of total RNA extracted from cells treated as above also showed a marked increase in COX-2 mRNA after 10, 50, and 100 ng/ml IGF-1 treatment (Figure 3B). IGF-1-induced phosphorylation of Akt and Erk1/2 occurred at 10 minutes and peaked at 30 minutes (Figure 3C). IGF-1 also induced COX-2 expression in a time-dependent manner, starting as early as 10 minutes with a peak at 6 hours (Figure 3C).
Figure 3.
IGF-1 activates COX-2 expression in RGM1 cells. A: Representative immunoblots with COX-2 and β-actin antibodies on total protein extracts showing an increase in COX-2 expression in RGM1 cells treated for 6 or 24 hours with IGF-1 at 10, 50, and 100 ng/ml or 100 ng/ml EGF compared with control. Quantification of immunoblots is expressed as relative density to β-actin (bottom). *P value <0.01 compared with control. B: RT-PCR with Cox-2 and β-actin primers performed using total RNA showing an increase in Cox-2 mRNA in RGM1 cells treated for 6 or 24 hours with IGF-1 at 10, 50, and 100 ng/ml or EGF at 100 ng/ml compared with medium only control. Semiquantification of RT-PCR is expressed as relative density to β-actin (bottom). C: Representative immunoblots with COX-2, phospho-Akt, total Akt, phopho-ERK1/2, total ERK1/2, and β-actin antibodies using total protein extracted from RGM1 cells treated with IGF-1 (10 ng/ml) showing a time-dependent increase in COX-2 expression, phospho-Akt, and phospho-ERK1/2 compared with medium only control.
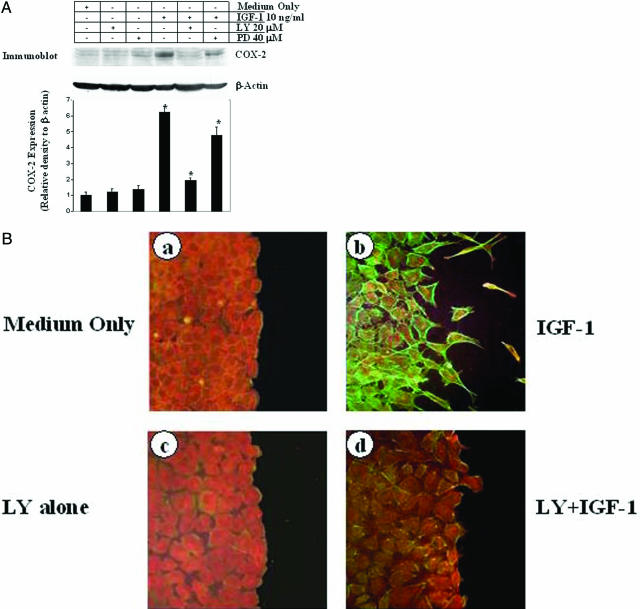
IGF-1 Activates COX-2 Expression and Promotes Actin Polymerization in RGM1 Cells through PI3K Signaling
Because IGF-1 activates both the Akt/PI3K and ERK/MEK/MAPK pathways in epithelial cells,34 we further examined the signaling pathway by which IGF-1 induces COX-2 expression in RGM1 cells. Pretreatment with PD98059 dramatically reduced IGF-1-induced ERK1/2 phosphorylation (data not shown) but reduced IGF-1-induced activation of COX-2 by only ∼27% compared with IGF-1 treatment alone (COX-2 expression relative to β-actin, 4.78 ± 0.49 versus 6.21 ± 0.36); (Figure 4A, lane 6). In contrast, pretreatment with LY294002 caused a dramatic reduction in Akt phosphorylation (data not shown) and ∼82% reduction in IGF-1-induced COX-2 expression compared with IGF-1 treatment alone (COX-2 expression relative to β-actin, 1.94 ± 0.16 versus 6.21 ± 0.36, P < 0.001) (Figure 4A, lane 5). This suggests that IGF-1-induced COX-2 expression is mediated mainly by PI3K signaling.
Figure 4.
IGF-1 activates COX-2 expression and promotes actin polymerization in RGM1 cells through PI3K signaling. A: Representative immunoblots with COX-2 and β-actin antibodies using total protein extracts from RGM1 cells showing that LY294002 inhibited IGF-1-induced COX-2 expression (lane 5), whereas PD980059 caused only partial inhibition of IGF-1-induced COX-2 expression (lane 6) compared with IGF-1 alone (lane 4), medium only control and vehicle controls (lanes 1, 2, and 3). The membrane was stripped and reprobed with β-actin to confirm equal loading. Quantification of COX-2 expression in immunoblots is shown at the bottom. *P value <0.001 as compared with IGF-1 alone. B: G-Actin (red) and F-actin (green) double staining of RGM1 cells 24 hours after treatment with 10 ng/ml IGF-1 (b), showing an increase in F-actin compared with medium only condition (a). Pretreatment of RGM1 cells with LY294002 alone did not change staining (c), whereas LY294002+IGF-1 caused a marked reduction in IGF-1-induced F-actin staining (d).
G-Actin polymerization into F-actin and the formation of stress fibers are crucial for cell migration.1,35 To determine the role of IGF-1 in promoting polymerization of G-actin into F-actin, a relative content of these two actin forms were assessed by double staining using Texas red-conjugated DNase1 for G-actin and Oregon Green-conjugated phalloidin for F-actin, as described in our previous study.28 Treatment with IGF-1 (10 ng/ml) caused a dramatic increase in F-actin in RGM1 cells compared with medium only treatment (Figure 4B, a and b), reflecting G-actin polymerization. RGM1 cells pretreated with LY294002 before treatment with IGF-1 had only a minimal increase in F-actin, suggesting that IGF-1 induced actin polymerization is mostly dependent on PI3K signaling (Figure 4B, c and d).
IGF-1-Induced RGM1 Cell Migration and Proliferation Are Dependent on PI3K Signaling and COX-2
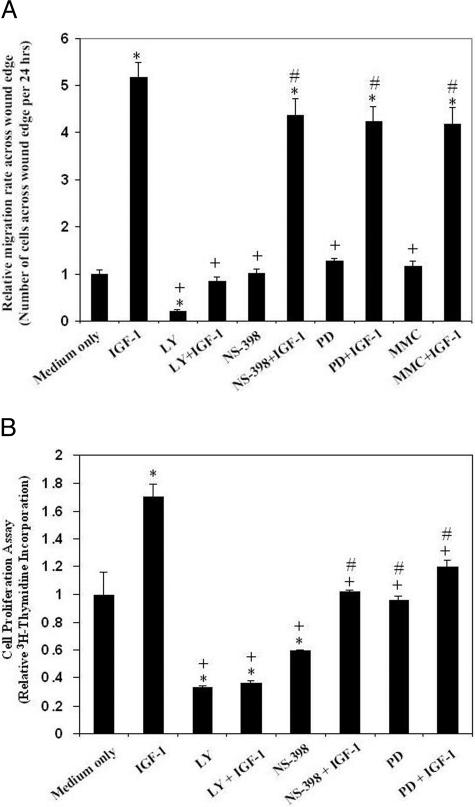
To examine further the roles of PI3K, COX-2, and MAPK in IGF-1-induced RGM1cell migration and proliferation, we performed RMG1 cell migration assay after treatment with IGF-1 or medium alone, in the presence or absence of PI3K inhibitor (LY294002), selective COX-2 inhibitor (NS-398), and MEK-1/2 inhibitor (PD98059). Pretreatment with LY294002 completely abolished IGF-1-induced RGM1 cell migration, whereas pretreatment with NS-398 and PD98059 caused a nonsignificant 15 and 18% reduction, respectively, in IGF-1-induced cell migration compared with controls (Figure 5A). This suggests that IGF-1-induced RGM1 cell migration is mostly dependent on PI3K signaling and not active COX-2 or MAPK signaling. LY294002 alone, reduced basal cell migration by 21% compared with medium alone, suggesting that cell migration at baseline is partly dependent on PI3K signaling. NS-398 and PD98059 alone did not significantly reduce basal cell migration compared with medium alone condition (Figure 5A). To determine the relative contribution of cell proliferation to RGM1 cell migration, cell proliferation was inhibited by preincubating with 2 μg/ml mitomycin C for 2 hours. Our previous studies demonstrated that at this concentration of mitomycin C, RGM1 cell proliferation at baseline is completely inhibited without adversely affecting cell migration.22 Treatment with mitomycin C caused a nonsignificant 19% reduction in IGF-1-induced cell migration compared with IGF-1 treatment alone, suggesting that a major part (81%) of IGF-1-induced RGM1 re-epithelization during the first 24 hours is attributable to cell migration and not proliferation (Figure 5A).
Figure 5.
Effect of IGF-1 on cell migration and proliferation: roles of PI3K, COX2, and mitogen-activated protein kinase. A: Quantitative data for RGM1 cell migration across wound edge. IGF-1 strongly induces cell migration. IGF-1-induced cell migration was significantly inhibited by pretreatment with LY294002 (LY) and partly inhibited by NS-398 and PD98059 (PD) compared with medium only and vehicle control. Inhibition of cell proliferation with 2 μg/ml mitomycin C only partly reduced IGF-1-induced RGM1 cell migration. *P value <0.001 compared with medium only control; +P value <0.001 compared with IGF-1 alone; #P value >0.05 compared with IGF-1 alone. All cell migration studies were done in triplicates and repeated twice; three random microscopic fields were selected for counting in each experiment (n = 9). B: Quantitative data for RGM1 cell proliferation using [3H]thymidine incorporation assay. IGF-1-induced cell proliferation was abolished by LY294002 and was partly inhibited by NS-398 compared with IGF-1 treatment alone, medium only, and vehicle control. *P value <0.005 compared with medium only control; +P value <0.05 compared with IGF-1 alone; #P value >0.05 compared with medium only control. All cell proliferation studies were done in triplicates and repeated twice (n = 9).
To determine whether IGF-1-induced cell proliferation is mediated through its activation of COX-2, PI3K, and MAPK signaling, [3H]thymidine incorporation assay was performed in RGM1 cells treated with IGF-1 alone or in the presence of LY294002, NS-398, or PD98059. IGF-1 treatment for 24 hours caused a 1.7-fold increase in [3H]thymidine incorporation compared with the medium only control. Pretreatment with LY294002 significantly reduced both the baseline and IGF-1-induced RGM1 cell proliferation (Figure 5B). Pretreatment with NS-398 and PD98059 also reduced both basal and IGF-1-induced proliferation but to a lesser extent than LY294002 (Figure 5B).
Discussion
Ulcer healing is a genetically programmed repair process that includes inflammation, cell proliferation, re-epithelialization, formation of granulation tissue, angiogenesis, interactions between various cells and matrix, and tissue remodeling, all resulting in scar formation. These events are controlled by cytokines, growth factors (eg, EGF, PDGF), and transcription factors activated by tissue injury in a spatially and temporally coordinated manner. These growth factors trigger mitogenic, motogenic, and survival signals using various signaling pathways.1,36,37,38,39 It has been demonstrated in various wound-healing models, eg, in injured ear, bones, brain, and dermal ulcers, that IGF-1 is up-regulated in a spatial and temporal manner in areas adjacent to the injured tissues.10,11,12,14,40 Our present study demonstrates for the first time that IGF-1 is locally up-regulated in the epithelial cells in proximity to gastric ulcer margin, which constitute a healing zone1,2,41,42 and is strongly expressed in cell re-epithelializing granulation tissue. This local IGF-1 up-regulation is attributable to gene activation because local IGF-1 mRNA level is also elevated in ulcerated gastric tissue.
To examine the functional consequences of local IGF-1 up-regulation, we used an in vitro model of gastric epithelial wound to study the actions of IGF-1 on cell migration and proliferation. We demonstrated that IGF-1 induces in RGM1 cells actin polymerization, cell migration, and proliferation and activates COX-2 in a PI3K signaling-dependent manner. Inhibition of PI3K signaling significantly reduced IGF-1-induced RGM1 cell proliferation and migration, whereas inhibition of COX-2 and MAPK only partly inhibited IGF-1-induced RGM1 cell proliferation and had no significant affect on cell migration, unlike our previous studies showing that NS-398 significantly inhibited bFGF-induced cell migration.43 This suggests a specific role for IGF-1 during gastric ulcer healing and that IGF-1-induced cell migration and proliferation is mediated mainly through PI3K pathway. When RGM1 cell proliferation was inhibited with mitomycin C, cell migration was minimally affected during the first 24 hours, indicating that a major effect of IGF-1 during the first 24 hours is on cell migration and not proliferation. These findings are consistent with previous observations using the same migration model to study effects bFGF, EGF, and HGF.22,43
Previous studies from others and our laboratories have shown that various growth factors such as EGF, bFGF, HGF, gastrin, and epiregulin may promote ulcer healing by up-regulating COX-2 expression.19,30,32,33,43,44 Selective inhibitors of COX-2 cause a delay in gastric ulcer healing and cellular restitution in vivo and in vitro.2,45 Our present study demonstrates for the first time a novel molecular mechanism of IGF-1 action on gastric epithelial cells—induction of COX-2 expression in a PI3K-dependent manner. Induction of COX-2 with resultant increase in generation of prostaglandin E2 may stimulate cell proliferation and ulcer healing. Our previous studies demonstrated that prostaglandin E2 exerts trophic and growth-promoting actions on gastric mucosa through transactivation of EGF receptor.27
Previous studies demonstrated that gastric ulceration activates expression of several growth factors including EGF and, as shown in this study, IGF-1. Our previous studies demonstrated the EGF-EGF-R-Erk2 pathway is critical for gastric ulcer healing46,47 and that blocking this pathway with specific EGF-R inhibitor dramatically delays ulcer healing.46 The role of IGF-1 in gastric ulcer healing is underscored by the fact that its deficiency in diabetic rats markedly delays ulcer healing.13,14 It is unclear whether ulceration-induced increase expression of both EGF and IGF-1 represent redundancy, a backup, or has a synergistic or an additive effect. Previous studies on wounded keratinocyte monolayer demonstrated that EGF and IGF-1 influence keratinocyte shape differently. Although IGF-1 stimulated membrane protrusion and facilitated cell spreading, EGF also induced contraction of keratinocytes. The effects of each growth factor on keratinocyte shape were mediated by distinct signal transduction pathways: EGF stimulated mitogen-activated protein kinase pathway, whereas IGF-1 stimulated the PI3K pathway. When added simultaneously, IGF-1 and EGF had an additive effect, providing faster and more complete wound epithelialization. These results showed that IGF-1 and EGF can influence different components of the keratinocyte migration machinery that determines the speed of wound epithelialization.48 Thus, it appears that effects of IGF-1 and EGF on cell migration are additive and mediated via two different signaling pathways: ERK2 for EGF and PI3K for IGF-1. In our present study, when IGF-1 and EGF were added together, they had a significant, additive affect on RGM1 cell migration. Thus, our present data demonstrate for the first time that this paradigm also applies to the healing of gastric epithelial cells as well.
In conclusion, we have demonstrated that IGF-1 is up-regulated in the gastric ulcer margin and that exogenous IGF-1 promotes gastric re-epithelization, proliferation, and COX-2 expression via the PI3K pathway. The up-regulation of COX-2 and PI3K signaling may be a key mechanism in mediating IGF-1-induced cell proliferation and migration, both essential components of re-epithelialization and ulcer healing.
Acknowledgments
We thank Drs. Lloyd Rucker and Robert Kaplan, University of California Irvine Medical Center and Veterans Administration Long Beach Healthcare System Department of Medicine, for their support of Resident Physician Research.
Footnotes
Address reprint requests to Andrzej S. Tarnawski, M.D., D.Sc., VA Long Beach Healthcare System, 5901 E. 7th St., Long Beach, CA 90822. E-mail: atarnawski@yahoo.com.
Supported by the Veterans Administration (Merit Review and Research Enhancement Award Program to A.S.T. and Merit Review Entry Program to J.C.).
T.A. was a visiting researcher from the Department of Surgery and Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; and T.T. was a visiting researcher from the Department of Gastroenterology, Osaka City University Graduate School of Medicine, Osaka, Japan.
References
- Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50(Suppl 1):S24–S33. doi: 10.1007/s10620-005-2803-6. [DOI] [PubMed] [Google Scholar]
- Tarnawski A. Molecular mechanisms of ulcer healing. Drug News Perspect. 2000;13:158–168. doi: 10.1358/dnp.2000.13.3.858438. [DOI] [PubMed] [Google Scholar]
- Basson MD, Modlin IM, Madri JA. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest. 1992;90:15–23. doi: 10.1172/JCI115828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Rabinovsky ED. The multifunctional role of IGF-1 in peripheral nerve regeneration. Neurol Res. 2004;26:204–210. doi: 10.1179/016164104225013851. [DOI] [PubMed] [Google Scholar]
- Costigan DC, Guyda HJ, Posner BI. Free insulin-like growth factor-1 (IGF-I) and IGF-II in human saliva. J Clin Endocrinol Metab. 1988;66:1014–1018. doi: 10.1210/jcem-66-5-1014. [DOI] [PubMed] [Google Scholar]
- Chaurasia OP, Marcuard SP, Seidel ER. Insulin-like growth factor I in human gastrointestinal exocrine secretions. Regul Pept. 1994;50:113–119. doi: 10.1016/0167-0115(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Rouyer-Fessard C, Gammeltoft S. Receptors for insulin-like growth factors I and II in rat gastrointestinal epithelium. Am J Physiol. 1988;254:G457–G462. doi: 10.1152/ajpgi.1988.254.3.G457. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Metalli VD, Alpini G, Onori P, Franchitto A, Barbaro B, Glaser SS, Francis H, Cantafora A, Blotta I, Attili AF, Gaudio E. The intrahepatic biliary epithelium is a target of the growth hormone/insulin-like growth factor 1 axis. J Hepatol. 2005;43:875–883. doi: 10.1016/j.jhep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Jennische E, Skottner A, Hansson HA. Dynamic changes in insulin-like growth factor I immunoreactivity correlate to repair events in rat ear after freeze-thaw injury. Exp Mol Pathol. 1987;47:193–201. doi: 10.1016/0014-4800(87)90074-8. [DOI] [PubMed] [Google Scholar]
- Edwall D, Prisell PT, Levinovitz A, Jennische E, Norstedt G. Expression of insulin-like growth factor I messenger ribonucleic acid in regenerating bone after fracture: influence of indomethacin. J Bone Miner Res. 1992;7:207–213. doi: 10.1002/jbmr.5650070212. [DOI] [PubMed] [Google Scholar]
- Garcia-Estrada J, Garcia-Segura LM, Torres-Aleman I. Expression of insulin-like growth factor I by astrocytes in response to injury. Brain Res. 1992;592:343–347. doi: 10.1016/0006-8993(92)91695-b. [DOI] [PubMed] [Google Scholar]
- Korolkiewicz RP, Tashima K, Fujita A, Kato S, Takeuchi K. Exogenous insulin-like growth factor (IGF-1) improves the impaired healing of gastric mucosal lesions in diabetic rats. Pharmacol Res. 2000;41:221–229. doi: 10.1006/phrs.1999.0581. [DOI] [PubMed] [Google Scholar]
- Kato S, Tanaka A, Ogawa Y, Kanatsu K, Seto K, Yoneda T, Takeuchi K. Effect of polaprezinc on impaired healing of chronic gastric ulcers in adjuvant-induced arthritic rats—role of insulin-like growth factors (IGF-1). Med Sci Monit. 2001;7:20–25. [PubMed] [Google Scholar]
- Coerper S, Wolf S, von Kiparski S, Thomas S, Zittel TT, Ranke MB, Hunt TK, Becker HD. Insulin-like growth factor I accelerates gastric ulcer healing by stimulating cell proliferation and by inhibiting gastric acid secretion. Scand J Gastroenterol. 2001;36:921–927. doi: 10.1080/003655201750305422. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Wang XE, Hirose M, Kivilioto T, Osada T, Miwa H, Oide H, Kitamura T, Yoneta T, Seto K, Sato N. Insulin-like growth factor I plays a role in gastric wound healing: evidence using a zinc derivative, polaprezinc, and an in vitro rabbit wound repair model. Aliment Pharmacol Ther. 1998;12:1131–1138. doi: 10.1046/j.1365-2036.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- Kato K, Chen MC, Nguyen M, Lehmann FS, Podolsky DK, Soll AH. Effects of growth factors and trefoil peptides on migration and replication in primary oxyntic cultures. Am J Physiol. 1999;276:G1105–G1116. doi: 10.1152/ajpgi.1999.276.5.G1105. [DOI] [PubMed] [Google Scholar]
- Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102:695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- Jones MK, Sasaki E, Halter F, Pai R, Nakamura T, Arakawa T, Kuroki T, Tarnawski AS. HGF triggers activation of the COX-2 gene in rat gastric epithelial cells: action mediated through the ERK2 signaling pathway. FASEB J. 1999;13:2186–2194. doi: 10.1096/fasebj.13.15.2186. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Kawano S, Tsuji S, Matsui H, Nakama A, Sawaoka H, Masuda E, Takei Y, Nagano K, Fusamoto H, Ohno T, Fukutomi H, Kamada T. RGM1, a cell line derived from normal gastric mucosa of rat. In Vitro Cell Dev Biol Anim. 1996;32:259–261. doi: 10.1007/BF02723056. [DOI] [PubMed] [Google Scholar]
- McCormack SA, Viar MJ, Johnson LR. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol. 1992;263:G426–G435. doi: 10.1152/ajpgi.1992.263.3.G426. [DOI] [PubMed] [Google Scholar]
- Netzer P, Halter F, Ma TY, Hoa N, Nguyen N, Nakamura T, Tarnawski AS. Interaction of hepatocyte growth factor and non-steroidal anti-inflammatory drugs during gastric epithelial wound healing. Digestion. 2003;67:118–128. doi: 10.1159/000071291. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Tominaga K, Watanabe T, Fujiwara Y, Oshitani N, Matsumoto T, Higuchi K, Tarnawski AS, Arakawa T. COX-2 is essential for EGF induction of cell proliferation in gastric RGM1 cells. Dig Dis Sci. 2003;48:2257–2262. doi: 10.1023/b:ddas.0000007860.87503.09. [DOI] [PubMed] [Google Scholar]
- Qiao M, Shapiro P, Kumar R, Passaniti A. Insulin-like growth factor-1 regulates endogenous RUNX2 activity in endothelial cells through a phosphatidylinositol 3-kinase/ERK-dependent and Akt-independent signaling pathway. J Biol Chem. 2004;279:42709–42718. doi: 10.1074/jbc.M404480200. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Percival MD. Effect of inhibitor time-dependency on selectivity towards cyclooxygenase isoforms. Biochem J. 1995;306:247–251. doi: 10.1042/bj3060247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futaki N, Takahashi S, Yokoyama M, Arai I, Higuchi S, Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- Chai J, Baatar D, Tarnawski A. Serum response factor promotes re-epithelialization and muscular structure restoration during gastric ulcer healing. Gastroenterology. 2004;126:1809–1818. doi: 10.1053/j.gastro.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Serrano ML, Romero A, Cendales R, Sanchez-Gomez M, Bravo MM. Serum levels of insulin-like growth factor-I and -II and insulin-like growth factor binding protein 3 in women with squamous intraepithelial lesions and cervical cancer. Biomedica. 2006;26:258–268. [PubMed] [Google Scholar]
- Horie-Sakata K, Shimada T, Hiraishi H, Terano A. Role of cyclooxygenase 2 in hepatocyte growth factor-mediated gastric epithelial restitution. J Clin Gastroenterol. 1998;27(Suppl 1):S40–S46. doi: 10.1097/00004836-199800001-00008. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Pai R, Halter F, Komurasaki T, Arakawa T, Kobayashi K, Kuroki T, Tarnawski AS. Induction of cyclooxygenase-2 in a rat gastric epithelial cell line by epiregulin and basic fibroblast growth factor. J Clin Gastroenterol. 1998;27(Suppl 1):S21–S27. doi: 10.1097/00004836-199800001-00005. [DOI] [PubMed] [Google Scholar]
- Komori M, Tsuji S, Sun WH, Tsujii M, Kawai N, Yasumaru M, Kakiuchi Y, Kimura A, Sasaki Y, Higashiyama S, Kawano S, Hori M. Gastrin enhances gastric mucosal integrity through cyclooxygenase-2 upregulation in rats. Am J Physiol. 2002;283:G1368–G1378. doi: 10.1152/ajpgi.00006.2002. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Sakamoto C, Matsuda K, Wada K, Uchida T, Noguchi H, Akamatsu T, Kasuga M. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Wang WJ, Chan JL, Wang LH. Differential requirements of the MAP kinase and PI3 kinase signaling pathways in Src- versus insulin and IGF-1 receptors-induced growth and transformation of rat intestinal epithelial cells. Oncogene. 2000;19:5385–5397. doi: 10.1038/sj.onc.1203911. [DOI] [PubMed] [Google Scholar]
- Banan A, Wang JY, McCormack SA, Johnson LR. Relationship between polyamines, actin distribution, and gastric healing in rats. Am J Physiol. 1996;271:G893–G903. doi: 10.1152/ajpgi.1996.271.5.G893. [DOI] [PubMed] [Google Scholar]
- Wong WM, Playford RJ, Wright NA. Peptide gene expression in gastrointestinal mucosal ulceration: ordered sequence or redundancy? Gut. 2000;46:286–292. doi: 10.1136/gut.46.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski A, Szabo IL, Husain SS, Soreghan B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J Physiol Paris. 2001;95:337–344. doi: 10.1016/s0928-4257(01)00046-8. [DOI] [PubMed] [Google Scholar]
- Jones MK, Tomikawa M, Mohajer B, Tarnawski AS. Gastrointestinal mucosal regeneration: role of growth factors. Front Biosci. 1999;4:D303–D309. doi: 10.2741/a428. [DOI] [PubMed] [Google Scholar]
- Tarnawski A, Halter F. Cellular mechanisms, interactions, and dynamics of gastric ulcer healing. J Clin Gastroenterol. 1995;21(Suppl 1):S93–S97. [PubMed] [Google Scholar]
- Gartner MH, Benson JD, Caldwell MD. Insulin-like growth factors I and II expression in the healing wound. J Surg Res. 1992;52:389–394. doi: 10.1016/0022-4804(92)90121-f. [DOI] [PubMed] [Google Scholar]
- Helpap B, Hattori T, Gedigk P. Repair of gastric ulcer. A cell kinetic study. Virchows Arch A Pathol Anat Histol. 1981;392:159–170. doi: 10.1007/BF00430818. [DOI] [PubMed] [Google Scholar]
- Helander HF. Morphological studies on the margin of gastric corpus wounds in the rat. J Submicrosc Cytol. 1983;15:627–643. [PubMed] [Google Scholar]
- Giap AQ, Tarnawski A, Hoa NT, Akotia V, Ma TY. NSAID inhibition of RGM1 gastric monolayer wound re-epithelialization: comparison of selective Cox-2 versus non-selective Cox inhibitors. Life Sci. 2002;70:3029–3037. doi: 10.1016/s0024-3205(02)01565-5. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Schuppan D, Drozdowicz D, Ptak A, Pawlik M, Nakamura T, Hahn EG. Involvement of cyclooxygenase (COX)-2 products in acceleration of ulcer healing by gastrin and hepatocyte growth factor. J Physiol Pharmacol. 2000;51:751–773. [PubMed] [Google Scholar]
- Halter F, Tarnawski AS, Schmassmann A, Peskar BM. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut. 2001;49:443–453. doi: 10.1136/gut.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai R, Ohta M, Itani RM, Sarfeh IJ, Tarnawski AS. Induction of mitogen-activated protein kinase signal transduction pathway during gastric ulcer healing in rats. Gastroenterology. 1998;114:706–713. doi: 10.1016/s0016-5085(98)70584-0. [DOI] [PubMed] [Google Scholar]
- Tarnawski AS, Pai R, Wang H, Tomikawa M. Translocation of MAP (Erk-1 and -2) kinases to cell nuclei and activation of c-fos gene during healing of experimental gastric ulcers. J Physiol Pharmacol. 1998;49:479–488. [PubMed] [Google Scholar]
- Haase I, Evans R, Pofahl R, Watt FM. Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci. 2003;116:3227–3238. doi: 10.1242/jcs.00610. [DOI] [PubMed] [Google Scholar]