Abstract
Diabetic nephropathy is associated with interstitial macrophage infiltrates, but their contribution to disease progression is unclear. We addressed this question by blockade of chemokine receptor (CCR)1 because CCR1 mediates the macrophage recruitment to the renal interstitium. In fact, when CCR1 was blocked with BL5923, a novel orally available CCR1 antagonist, the interstitial recruitment of ex vivo labeled macrophages was markedly decreased in uninephrectomized male db/db mice with advanced diabetic nephropathy. Likewise, BL5923 (60 mg/kg, twice a day) orally administered from months 5 to 6 of life reduced the numbers of interstitial macrophages in uninephrectomized db/db mice. This was associated with reduced numbers of Ki-67 proliferating tubular epithelial and interstitial cells, tubular atrophy, and interstitial fibrosis in uninephrectomized db/db mice. Glomerular pathology and proteinuria were not affected by the CCR1 antagonist. BL5923 reduced renal mRNA expression of Ccl2, Ccr1, Ccr2, Ccr5, transforming growth factor-β1, and collagen I-α1 when compared with untreated uninephrectomized male db/db mice of the same age. Thus, we identified a previously unrecognized role for interstitial macrophages for tubulointerstitial injury, loss of peritubular microvasculature, interstitial inflammation, and fibrosis in type 2 diabetic db/db mice. These data identify oral treatment with the CCR1 antagonist BL5923 as a potential therapy for late-stage diabetic nephropathy.
Diabetic nephropathy is worldwide a leading cause of end-stage renal failure because available therapies can slow but not prevent disease progression.1,2,3,4 Therefore, development of new therapies that target additional disease mechanisms of diabetic nephropathy are greatly needed. Recent experimental evidence links the progression of diabetic nephropathy to intrarenal inflammation and leukocytic cell infiltrates.5,6,7,8,9 For example, mycophenolate mofetil, methotrexate, and irradiation have recently been shown to reduce urinary albumin excretion and glomerulosclerosis in rats with streptozotocin-induced diabetic nephropathy.10,11,12 However, the molecular and cellular mechanisms of intrarenal inflammation in diabetic nephropathy remain poorly characterized. Clinical studies have noted increased serum levels of acute phase markers of inflammation in patients with diabetic nephropathy, but this may not represent intrarenal inflammation.13,14 In this regard, the finding that patients with diabetic nephropathy excrete high levels of the CC-chemokine monocyte chemoattractant protein 1 (CCL2, formerly MCP-1) in the urine may be more specific.15 In fact, glomerular and interstitial macrophage infiltrates have been recognized in human biopsy samples and rodent models of diabetic nephropathy.10,12,16,17,18,19,20,21 Macrophage infiltrates are usually associated with intrarenal inflammation,22,23 but their functional role for the progression of diabetic nephropathy remains uncertain. Interestingly, in intercellular adhesion molecule-1- or Ccl2-deficient diabetic mice, lower glomerular and interstitial macrophage counts were associated with less glomerular and tubulointerstitial injury.24,25 The adhesion molecule intercellular adhesion molecule-1 and the CC-chemokine CCL2 are both involved in the complex multistep process of leukocyte recruitment from intravascular to extravascular compartments, ie, glomeruli and renal interstitium.26 In experimental diabetic nephropathy, macrophages recruit sequentially to these two compartments of the kidney. Although glomerular macrophages appear at an early stage, macrophages recruit to the interstitial compartment only at an advanced stage of diabetic nephropathy.19 Targeting a molecule that specifically mediates the recruitment of macrophages to the renal interstitium would represent a powerful tool to dissect the role of interstitial macrophages in diabetic nephropathy.
We and others recently found that the chemokine receptor (CCR)-1 is critical for the recruitment of interstitial macrophages.27 Lack or blockade of CCR1 enables macrophage adhesion and transendothelial diapedesis in mice and contributes to macrophage activation in inflammatory cytokine environments.28 Thus, CCR1 blockade may represent an appropriate tool to study the contribution of interstitial macrophages to diabetic nephropathy.
We hypothesized that blocking CCR1-dependent interstitial macrophage recruitment would reduce tubulointerstitial inflammation and tubular injury in diabetic nephropathy, a hypothesis supported by our results. We used db/db mice as a model of type 2 diabetes because db/db mice develop kidney disease with similarities to human diabetic nephropathy.29 Experimentally, uninephrectomy was used to enhance the development of advanced diabetic nephropathy.30 Uninephrectomized type 2 diabetic db/db mice were treated with BL5923, a recently developed small molecule antagonist with a high specificity for human and murine CCR1.31 BL5923 has almost identical activity on human and murine CCR1 and allows oral administration, two major pharmacological advantages over previously available CCR1 antagonists.31
Materials and Methods
Animal Studies
Male 5-week-old C57BLKS db/db or C57BLKS wild-type mice were obtained from Taconic (Ry, Denmark) and housed in filter top cages with a 12-hour dark/light cycle and unlimited access to food and water for the duration of the study. Cages, bedding, nestlets, food, and water were sterilized by autoclaving before use. All experimental procedures were approved by the local government authorities. At the age of 6 weeks, mice underwent uninephrectomy (1K mice) or sham surgery (2K mice) through a 1-cm flank incision as previously described.30 In mice of the sham surgery groups, the kidney was left in situ. 1K db/db mice were divided in three groups that received either BL5923 (60 mg/kg, twice a day) in the vehicle (0.5% hydroxyethyl cellulose) (Sigma-Aldrich, Steinheim, Germany), vehicle only, or nil by oral gavage from 5 months of age. Treatment was continued for 4 weeks, when tissues were obtained for histopathological evaluation. Urine samples were obtained at monthly intervals and analyzed for albumin/creatinine ratios as described.28 Blood glucose levels were determined at monthly intervals using a glucometer (Accu Check Sensor; Roche, Mannheim, Germany). White blood counts were determined with a Coulter counter (Beckman Coulter GmbH, Krefeld, Germany).
Immunohistochemistry
All immunohistological studies were performed on paraffin-embedded sections as described.28 The following rat and rabbit antibodies were used as primary antibodies: rat anti-Mac2 (glomerular macrophages, 1:50; Cederlane, Ontario, ON, Canada), rat anti-F4/80 (interstitial macrophages, 1:50; Serotec, Oxford, UK), rat anti-CD3 (lymphocytes, 1:50; Serotec), anti-Ki-67 (cell proliferation, 1:25; Dianova, Hamburg, Germany), anti-mMECA-32 (endothelial cells, 1:50; Iowa Hybridoma Bank, Iowa City, IA), and ND anti-mCCL5 (1:50; Peprotech, Rocky Hill, NJ).
Histopathological Evaluation
From each mouse parts of the kidneys were fixed in 10% formalin in phosphate-buffered saline and embedded in paraffin. Four-μm sections were stained with periodic acid-Schiff reagent or silver following the instructions of the supplier (Bio-Optica, Milano, Italy). Glomerular sclerotic lesions were assessed using a semiquantitative score by a blinded observer as follows: 0 = no lesion, 1 = <25% sclerotic, 2 = 25 to 49% sclerotic, 3 = 50 to 74% sclerotic, and 4 = 75 to 100% sclerotic, respectively. Fifteen glomeruli were analyzed per section. The indices for interstitial volume, interstitial collagen deposition, tubular cell damage, and tubular dilatation were determined as described previously.28 Interstitial cell counts were determined in 15 high-power fields (×400) by a blinded observer.
Real-Time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Kidney segments from each animal were snap-frozen in liquid nitrogen and stored at −80°C. RNA preparation from cultured cells and kidneys as well as real-time RT-PCR were performed as described.28 Controls consisting of ddH2O were negative for target and housekeeper genes. Oligonucleotide primers (300 nmol/L) and probes (100 nmol/L) were used as listed in Table 1. Primers and probes were from PE Biosystems, Weiterstadt, Germany.
Table 1.
Primers and Probes Used for Real-Time RT-PCR
| Target | Sequence |
|---|---|
| Ccl2 | Predeveloped TaqMan assay reagent from PE Biosystems |
| Ccl5 | Predeveloped TaqMan assay reagent from PE Biosystems |
| Ccr1 | Forward primer: 5′-TTAGCTTCCATGCCTGCCTTATA-3′ |
| Reverse primer: 5′-TCCACTGCTTCAGGCTCTTGT-3′ | |
| Probe: 6 FAM-5′-ACTCACCGTACCTGTAGCCCTCATTTCCC-3′ | |
| Ccr2 | Forward primer: 5′-CCTTGGGAATGAGTAACTGTGTGA-3′ |
| Reverse primer: 5′-ACAAAGGCATAAATG-ACAGGATTAATG-3′ | |
| Probe: 6 FAM-5′-TGACAAGCACTTAGACCAGGCCATGCA-3′ | |
| Ccr5 | Forward primer: 5′-CAAGACAATCCTGATCGTGCAA-3′ |
| Reverse primer: 5′-TCCTACTCCCAAGCTGCATAGAA-3′ | |
| Probe: 6 FAM-5′-TCTATACCCGATCCACAGGAGAACATGAAGTTT-3′ | |
| Tgf-β1 | Forward primer: 5′-CACAGTACAGCAAGGTCCTTGC-3′ |
| Reverse primer: 5′-AGTAGACGATGGGCAGTGGCT-3′ | |
| Probe: 6 FAM-5′-GCTTCGGCGTCACCGTGCT-3′ | |
| Collagen I-α1 | Forward primer: 5′-TGCTTTCTGCCCGGAAGA-3′ |
| Reverse primer: 5′-GGGATGCCATCTCGTCCA-3′ | |
| Probe: 6 FAM-5′-CCAGGGTCTCCCTTGGGTCCTACATCT-3′ | |
| 18s rRNA | Predeveloped TaqMan assay reagent from PE Biosystems |
In Vivo Assay of Renal Macrophage Recruitment
F4/80-positive macrophages were prepared by immunomagnetic selection from spleens of db/db mice as previously described.28 Purity of isolated cells was verified by flow cytometry. Separated cells were labeled with PKH26 (Red Fluorescent Cell Linker kit; Sigma-Aldrich Chemicals), and labeling efficacy was assessed by flow cytometry. F4/80 macrophages (3.5 × 105) in 200 μl of isotonic saline were injected into the tail vein of 6-month-old db/db mice that had received a single dose of either BL5923 or vehicle 3 hours before injection. Renal tissue was obtained after 3 hours, snap-frozen, and prepared for fluorescence microscopy. The number of interstitial fluorescent cells was determined in 15 high-power fields.
Determination of BL5926 Blood Levels
Blood samples (45 μl) were spiked with an internal standard (5 μl) and extracted with 200 μl of acetonitrile. After centrifugation, 220 μl of the supernatant were dried and redissolved in 60 μl of methanol and 40 μl of 0.1% formic acid. The solution was centrifuged, and 10 μl of the supernatant were analyzed by high-performance liquid chromatography/mass spectrometry using the Rheos LC high-performance liquid chromatography system (Erca-tech AG, Bern, Switzerland). Eluent A was water with 1.5% formic acid plus 0.02% trifluoroacetic acid (TFA); eluent B was acetonitrile/methanol (50:50, v/v) with 1.5% formic acid plus 0.02% TFA. Column efflux was directly introduced into the ion source of a Finnigan Quantum Ultra MS detector. Quantitative analysis was performed by selected ion monitoring over the respective quasi-molecular ions. The calibration curve was performed in triplicate. Data from blood samples were calculated along the calibration curve and expressed in ng/ml.
Cell Culture Experiments
J774 murine macrophages (American Type Culture Collection, Rockville, MD) were grown in RPMI 1640 medium (GIBCO/Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C supplied with 5% CO2/air. A proximal tubular epithelial cell line was maintained in Dulbecco’s modified Eagle’s medium (GIBCO/Invitrogen) containing 10% fetal calf serum and 1% penicillin-streptomycin.32 Cells were kept in medium with or without fetal calf serum for 24 hours before incubation with BL5923 (10 or 50 μg/ml). Proliferation of J774 murine macrophages and proximal tubular epithelial cells was assessed after 72 hours using CellTiter 96 proliferation assay by adding 20 μl of CellTiter 96 Aqueous One solution to each well and was kept for 1.5 hours at 37°C (Promega, Mannheim, Germany). The optical density was measured at 492 nm.
Statistical Analysis
Data are presented as mean ± SEM. Intravital microscopy data were analyzed using one-way analysis of variance followed by Student-Newman-Keuls test, using SigmaStat Software (Jandel Scientific, Erkrath, Germany). Comparison of groups was performed using analysis of variance and posthoc Bonferroni’s correction was used for multiple comparisons. A value of P < 0.05 was considered statistically significant.
Results
Uninephrectomy Accelerates Diabetic Nephropathy and Renal Ccr1 mRNA Expression in db/db Mice
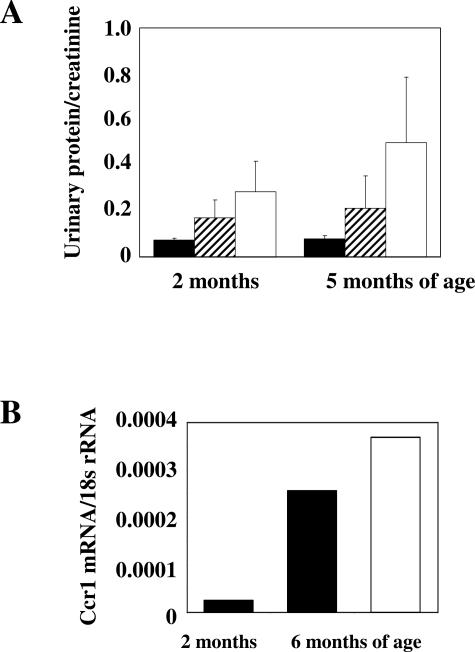
Albuminuria is the first functional marker of diabetic nephropathy in humans and db/db mice. In fact, 2-month-old db/db mice revealed increased albuminuria as compared with wild-type mice of the same age (Figure 1A). Uninephrectomy performed in db/db mice (1K) at 6 weeks of age was associated with higher albuminuria levels as compared with sham-operated db/db mice (2K). The nephrectomy-related impact on albuminuria further increased until 5 months of age, consistent with uninephrectomy accelerating diabetic nephropathy in db/db mice.30 Uninephrectomy may simply increase albuminuria by hyperfiltration and not necessarily via a CCR1-dependent mechanism. Therefore, we examined the renal expression of CCR1 in 1K and 2K db/db mice. We have previously shown that intrinsic renal cells do not express the chemokine receptor CCR1 in the mouse kidney and that renal CCR1 expression originates from infiltrating macrophages and T cells.33 Because appropriate antibodies that allow detection of CCR1 protein by cell fluorescence or immunostaining in mice are not available, we used real-time RT-PCR to determine the expression of Ccr1 mRNA in kidneys of db/db mice. Kidneys of 2-month-old 2K db/db mice showed low Ccr1 mRNA expression that markedly increased until 6 months of age (Figure 1B). Renal Ccr1 mRNA expression further increased in 1K db/db mice.
Figure 1.
Effect of uninephrectomy on diabetic nephropathy of db/db mice. A: Urinary albumin/creatinine ratios were determined in 2K wild-type mice (black bars), 2K db/db mice (hatched bars), and 1K db/db mice (white bars). Values represent means ± SEM from 7 to 10 mice in each group. B: Quantitative real-time RT-PCR analysis was performed on total cDNA derived from kidneys of 2- or 6-month-old 2K db/db mice (black bars) or 6-month-old 1K db/db mice (white bars). The cDNA was amplified using primers specific for mCCR1 for 40 PCR cycles. The data shown are derived from pooled cDNA samples from 6 to 10 mice of each group and are expressed as ratio to respective 18s rRNA expression.
BL5923 Reduces Recruitment of Macrophages to the Renal Interstitium of 1K db/db Mice
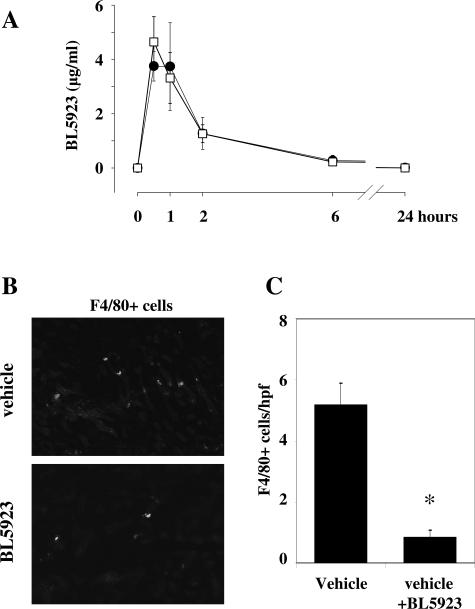
Next, we tested whether the pharmacokinetic profile of the CCR1 antagonist BL5923 is affected by uninephrectomy and whether BL5923 can block macrophage recruitment to the renal interstitium of 1K db/db mice. The pharmacokinetic data show that the half-life of BL5923 is not prolonged in 1K db/db mice. The plasma compound levels were identical in sham-operated and uninephrectomized db/db mice (Figure 2A). Next, we performed cell transfer studies with ex vivo fluorescently labeled F4/80-positive monocytes into 6-month-old 1K db/db mice that had been pretreated with a single dose of either BL5923 or vehicle. Three hours after injection, F4/80 cells were found to localize to the interstitial compartment of 1K db/db mice (Figure 2B). Pretreatment with BL5923 significantly reduced the numbers of labeled F4/80 cells that infiltrated into the renal interstitium of 1K db/db mice (Figure 2C). Next, we assessed white blood counts in mice treated with BL5923. A single dose of BL5923 decreased white blood counts in sham-operated and uninephrectomized db/db mice [6.1 ± 0.6 × 103/μl versus 3.2 ± 0.8 × 103/μl (sham-operated) or versus 3.4 ± 0.6 × 103/μl (uninephrectomized), P < 0.001, respectively]. These data provide the rationale for using BL5923 to block interstitial macrophage recruitment in 1K db/db mice, an accelerated model of diabetic nephropathy.
Figure 2.
Pharmacokinetic profile of BL5923 and recruitment of monocytes into the renal interstitium of db/db mice. A: BL5923 was applied once orally at 60 mg/kg to groups of six 2K db/db mice (closed circles) or 1K db/db mice (open squares). Blood samples were obtained at 30 minutes and 1 hour, 2 hours, 6 hours, and 24 hours, and the plasma level of BL5923 were determined by HLPC/MS and expressed as mean ± SEM in μg/ml. B: 1K db/db mice 6 months of age were injected intravenously with PKH26-labeled F4/80 monocytes isolated from spleens of donor db/db mice. Recipient mice were pretreated with a single dose of either vehicle or BL5923 before injection of the respective cells, and kidneys were obtained 3 hours after injection of cells and examined by fluorescence microscopy. Fluorescence-labeled cells locate to the renal interstitium. C: Cell counts for interstitial labeled F4/80 monocytes were determined by fluorescence microscopy from 15 high-powered fields. Values are means ± SEM. *P < 0.001. Original magnifications, ×400.
BL5923 Reduces Interstitial Macrophage Counts and Tubulointerstitial Injury in 1K db/db Mice
Because BL5923 can block interstitial macrophage recruitment in 1K db/db mice, BL5923 treatment may have beneficial effects on the progression of diabetic nephropathy associated with tubulointerstitial injury and interstitial fibrosis. We initiated oral administration of BL5923 (60 mg/kg, twice a day) or vehicle at 5 months of age in 1K db/db mice. Treatment was continued for 4 weeks, when urine samples and tissues were collected for the assessment of diabetic nephropathy. During that period BL5923 treatment did not significantly affect blood glucose levels or body weight, which were both markedly elevated in all groups of db/db mice as compared with nondiabetic wild-type mice (Figure 3, A and B).
Figure 3.
Blood glucose levels, body weight, and proteinuria in db/db and wild-type mice. Blood glucose levels (A) and body weight (B) were determined at monthly intervals in 2K wild-type mice (open triangles), 2K db/db mice (black triangles), and 1K db/db mice (nil: black squares, dashed line; vehicle: black squares; BL5923: open squares). Values represent means ± SEM.
Glomerular Injury
At 6 months of age, diabetic glomerulosclerosis was more prominent in 1K db/db mice as compared with 2K db/db mice (Table 2, Figure 4). BL5923 had no effect on glomerulosclerosis or urinary albumin/creatinine ratios of 6-month-old db/db mice (Table 2, Figure 4). BL5923 did not affect the number of Mac-2-positive glomerular macrophages or Ki-67-positive proliferating glomerular cells (Table 2, Figure 4). Blood urea nitrogen levels were comparable in vehicle- and BL5923-treated 1K db/db mice.
Table 2.
Serum, Urinary, and Histological Findings in Sham-Operated (2K) and Uninephrectomized (1K) Mice
| 2K
|
1K
|
||||
|---|---|---|---|---|---|
| Wild-type + nil (n = 7) | db/db + nil (n = 7) | db/db + nil (n = 9) | db/db + vehicle (n = 7) | db/db + BL5923 (n = 8) | |
| Renal function | |||||
| Urine albumin/creatinine | 0.1 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.9 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| BUN (mg/dl) | 27 ± 5 | 42 ± 18 | 51 ± 10 | 37 ± 6 | 37 ± 7 |
| Glomerulosclerosis score | |||||
| 0 (no lesion) | 91 ± 5 | 12 ± 6 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 1 (1 to 24%) | 6 ± 2 | 20 ± 2 | 4 ± 5 | 3 ± 4 | 3 ± 5 |
| 2 (25 to 49%) | 3 ± 4 | 31 ± 9 | 23 ± 8 | 27 ± 4 | 29 ± 6 |
| 3 (50 to 74%) | 0 ± 0 | 26 ± 11 | 32 ± 3 | 39 ± 5 | 33 ± 4 |
| 4 (75 to 100%) | 0 ± 0 | 11 ± 3 | 40 ± 10 | 31 ± 9 | 34 ± 7 |
| Cellular response (cells/glomerulus or hpf) | |||||
| Glomerular Ki-67+ | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.4 | 0.9 ± 0.2 | 0.8 ± 0.3 |
| Mac-2+ | 0.2 ± 0.1 | 1.7 ± 0.1 | 2.4 ± 0.7 | 2.6 ± 0.3 | 2.2 ± 0.5 |
| Interstitial F4/80+ | 2.8 ± 0.3 | 8.4 ± 1.3 | 14.4 ± 2.1 | 14.1 ± 2.2 | 2.3 ± 1.5* |
| Ki-67+ | 0.7 ± 0.2 | 1.4 ± 0.3 | 3.9 ± 1.5 | 3.8 ± 1.2 | 0.7 ± 0.3* |
| Tubular Ki-67+ | 0.5 ± 0.2 | 1.7 ± 0.2 | 5.7 ± 1.5 | 5.0 ± 0.4 | 2.2 ± 0.5* |
| Peritubular capillaries (capillary cross sections/hpf) | |||||
| MECA-32+ | 123 ± 13 | 75 ± 11 | 81 ± 10 | 79 ± 9 | 103 ± 11* |
Values are means ± SEM, *P < 0.05 versus vehicle.
Figure 4.
Renal histopathology. Renal sections from mice of all groups were stained with periodic acid-Schiff solution or for the indicated markers as described in Materials and Methods. For quantification, see Table 1. Original magnifications, ×200 (F4/80+ and Ki-67) and ×400 (PAS).
Tubulointerstitial Injury
Indices for flattened or necrotic tubular cells, tubular dilatation, interstitial matrix, and interstitial volume as markers of tubulointerstitial damage and renal fibrosis were assessed in mice of all groups by morphometry. BL5923 reduced all these markers in 1K db/db mice although the reduction of interstitial collagen deposits did not reach statistical significance (Figure 5). Interstitial disease in vehicle-treated 1K db/db mice was associated with a robust increase of interstitial F4/80-positive macrophages, which were markedly reduced by BL5923 (Figure 4, Table 2). CD3-positive lymphocytes were absent in kidneys of 6-month-old db/db mice (not shown). The reduction of interstitial macrophages was associated with reduced numbers of Ki-67-positive proliferating tubular epithelial cells as well as proliferating cells in the interstitial compartment (Table 2, Figure 4). BL5923 prevented the reduction in MECA32-positive peritubular capillary cross sections, which was observed in vehicle-treated 1K db/db mice (Table 2). These findings show that blocking CCR1-dependent interstitial macrophage recruitment preserves tubulointerstitial injury in db/db mice.
Figure 5.
Renal fibrosis. A: Renal sections from mice of all groups were stained with silver. Images illustrate representative sections of kidneys from the respective groups at 6 months of age. B: Morphometric analysis of cortical renal sections was performed as described in Materials and Methods. Values represent means ± SEM of the respective index from 7 to 10 mice in each group. WT, wild type. *P < 0.05 versus vehicle-treated 1K db/db mice. Original magnifications, ×100.
CCR1 Blockade Reduces Renal Expression of Proinflammatory Mediators in 1K db/db Mice
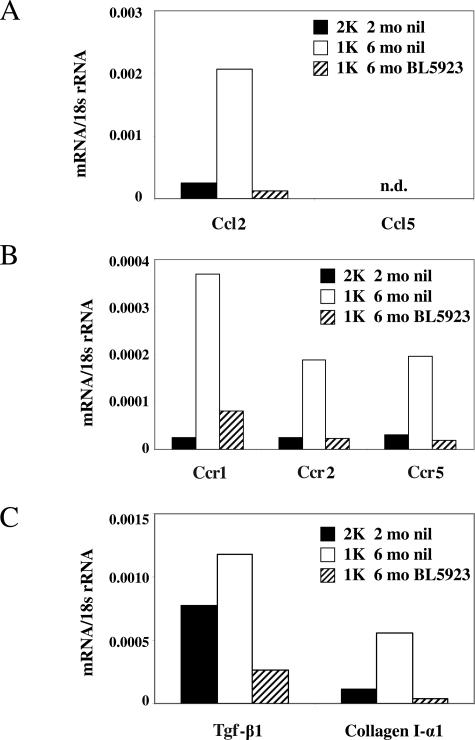
Macrophages are a major source of proinflammatory and profibrotic mediators in renal injury. Therefore, we assessed whether the BL5923-induced reduction of interstitial macrophage infiltrates affects the expression levels of proinflammatory mediators in kidneys of 1K db/db mice. We used real-time RT-PCR to quantify the mRNA expression of chemokines and chemokine receptors that drive renal leukocyte recruitment in chronic kidney disease.34 BL5923 reduced mRNA levels of Ccl2, Ccr1, Ccr2, and Ccr5 in kidneys of 6-month-old 1K db/db mice (Figure 6). Ccl5 mRNA expression levels were undetectable in kidneys of all groups. In addition, BL5923 reduced renal mRNA expression of transforming growth factor (Tgf)-β1 and collagen I-α1, two markers of interstitial fibrosis in mice (Figure 6). These data indicate that blocking CCR1-dependent interstitial macrophage recruitment reduces the renal expression of proinflammatory mediators, ie, Ccl2, Ccr1, Ccr2, Ccr5, and markers of interstitial fibrosis, eg, Tgf-β1 and collagen I-α1 in 1K db/db mice.
Figure 6.
Renal mRNA expression of proinflammatory mediators and markers of fibrosis. mRNA expression for the CC-chemokines Ccl2 and Ccl5 (A), their respective CC-chemokine receptors Ccr1, Ccr2, and Ccr5 (B), Tgf-β1, and collagen I-α1 (C) was determined by real-time RT-PCR using total renal RNA pooled from 6 to 10 mice of each group. mRNA levels for each group of mice are expressed per respective 18s rRNA expression.
CCR1 Blockade Inhibits the Proliferation of J774 Cells but Not of Tubular Epithelial Cells
Our finding that BL5923 reduces the number of proliferating cells in the tubular as well as interstitial compartment raises the question whether these effects are directly mediated via CCR1 on these cells. We used J774 cells, a murine monocyte/macrophage cell line, and a murine tubular epithelial cell line32 to assess Ccr1 mRNA expression and to study the impact of BL5923 on the proliferation rate of these cells. Ccr1 mRNA was not detectable in cultured tubular epithelial cells, whereas J774 expressed Ccr1 mRNA at high levels (Figure 7A). The proliferation rate of both cell lines was low within 48 hours in the absence of fetal calf serum but markedly increased when fetal calf serum was added to the culture dishes (Figure 7B). When BL5923 was added, the proliferation rate of Ccr1-positive J774 cells significantly declined. By contrast, BL5923 had no effect on the proliferation rate of Ccr1-negative tubular epithelial cells.
Figure 7.
BL5923 and the proliferation of J774 macrophages or tubular epithelial cells. A: The Ccr1 mRNA expression levels were determined in cultured J774 macrophages and tubular epithelial cells as described in Materials and Methods. Data are expressed as means ± SEM of the ratio of Ccr1 mRNA and the respective 18s rRNA level. B: Proliferation of cultured J774 macrophages and tubular epithelial cells was assessed after 72 hours using the CellTiter 96 proliferation assay as described in Materials and Methods. Data are expressed at means ± SEM of the optical density (O.D.) read at a wavelength of 492 nm. *P < 0.05 versus medium + 10% fetal calf serum (FCS).
Discussion
We used a CCR1 antagonist to block interstitial macrophage recruitment in 1K db/db mice, an accelerated model for advanced nephropathy of type 2 diabetes. CCR1 blockade reduced interstitial macrophage infiltrates, most likely by interfering with macrophage adhesion to activated endothelial cells of peritubular capillaries in the renal interstitium. Using intravital microscopy we have recently shown that leukocyte adhesion is impaired in Ccr1-deficient mice or C57BL/6 mice treated with BX471, another CCR1 antagonist.28 BL5923, the orally available CCR1 antagonist used here, showed comparable activity in 1K db/db mice in blocking the recruitment of fluorescently labeled macrophages into the renal interstitial compartment of 1K db/db mice. Adhesion is an early and critical event in the multistep process of leukocyte evasion from the circulation.26 Thus, consistent with the low numbers of fluorescently labeled intrarenal macrophages in BL5923-treated 1K db/db mice, labeled macrophages could not retain at activated peritubular capillaries but were carried away with the peritubular blood flow. In addition, we found that CCR1 blockade reduces the proliferation of murine monocytes/macrophages in the presence of serum. This may represent another mechanism by which BL5923 reduced the number of interstitial macrophages in db/db mice. In fact, the number of interstitial proliferating cells was reduced in BL5923-treated db/db mice. Furthermore, we found that a single dose of the CCR1 antagonist reduces the white blood count in db/db mice. Chemokine receptors not only mediate leukocyte recruitment to peripheral tissues but are also involved in mobilizing monocytes from the bone marrow into the intravascular compartment.35 The role of CCR1 in this process needs to be explored in future studies. Together, CCR1 blockade is modulating the number of renal interstitial macrophages by multiple mechanisms, eg, lowering the number of white blood cells in the intravascular compartment, blocking extravasation into the renal interstitial compartment, and inhibiting macrophage proliferation.
Selectively manipulating the number of interstitial macrophages in 1K db/db mice should allow determination of their functional role for the progression of diabetic nephropathy. Most interestingly, reduced numbers of interstitial macrophages were associated with improved peritubular vasculature and the extent of tubulointerstitial injury and interstitial fibrosis, all of which are important predictors of disease progression in diabetic nephropathy.36,37 These data indicate that the presence of macrophages in diabetic nephropathy contributes to renal injury, a mechanism that may be referred to as “inflammation.”6,7,9 Inflammatory or anti-inflammatory phenotypes of renal macrophages are difficult to determine by immunostaining but can best be determined by their function in vivo.38 For example, interstitial macrophages produce large amounts of proinflammatory mediators, ie, cytokines and chemokines, which add to the mediators produced by renal cells, ie, in a positive amplification loop.33,39 This observation made in nondiabetic types of kidney disease is likely also to apply to diabetic nephropathy in humans because interstitial macrophage infiltrates are common in diabetic nephropathy16 and patients with diabetic nephropathy excrete high levels of CC-chemokines into the urine. For example, the urinary excretion of CCL2 (formerly monocyte chemoattractant protein-1) indicates intrarenal inflammation.15,40 Chemokine expression involves activation of protein kinase C in renal cells as well as immune cell infiltrates. Therapeutic intervention targeting protein kinase C can disrupt this positive amplification loop by reducing renal chemokine expression, subsequent recruitment of immune cells, and tubular injury in experimental and human diabetic nephropathy.20,41 However, protein kinase C blockade cannot address the role of single chemokine ligands in the inflammatory lesion in diabetic nephropathy. Chow and colleagues25 addressed the role of CCL2 in the pathogenesis of experimental diabetic nephropathy by inducing type I diabetes in Ccl2-deficient mice. Ccl2-deficient mice were primarily protected from renal injury after streptozotocin injection, which was associated with markedly reduced macrophage infiltrates in the glomerular and tubulointerstitial compartments. Although this study supports an important role of CCL2 in the pathogenesis of experimental diabetic nephropathy, the role of interstitial macrophages for progression of diabetic nephropathy remains unclear. The selective recruitment of a certain immune cell subset can better be achieved by delayed blockade of a single chemokine receptor that mediates the recruitment of this cell type to the compartment of interest, ie, CCR1 for the recruitment of macrophages to the renal interstitium. Our data indicate that interstitial macrophages are a major source and trigger of intrarenal cytokine and chemokine production in experimental diabetic nephropathy. Blocking CCR1-dependent interstitial macrophage recruitment reduced the mRNA expression of the CC-chemokine CCL2 in kidneys of 1K db/db mice, for which a crucial role in the progression of diabetic nephropathy was recently demonstrated.15,25 As a consequence of reduced intrarenal chemokine signaling and CCR1 blockade, subsequent leukocyte recruitment was impaired in association with reduced renal mRNA expression of the proinflammatory chemokine receptors Ccr1, Ccr2, and Ccr5, factors not expressed by nonimmune renal cells in vivo.
Loss of peritubular capillaries is a well-known marker for advanced interstitial injury and is thought to contribute to renal ischemia, a major stimulus for fibroblast proliferation and production of extracellular matrix.42 In fact, blocking CCR1-dependent interstitial macrophage recruitment was also associated with less interstitial fibrosis. This was indicated by less renal Tgf-β1 and collagen I-α1 mRNA expression as well as interstitial collagen deposits in BL5923-treated 1K db/db mice. Direct effects of BL5923 on intrinsic renal cells in the renal tubulointerstitium are unlikely. We did not observe direct effects of BL5923 on tubular epithelial cells in vitro because these cells lack CCR1 expression. Together, these data identify a previously unrecognized role for interstitial macrophages for tubulointerstitial injury, loss of peritubular microvasculature, interstitial inflammation, and fibrosis in type 2 diabetic db/db mice.
Acknowledgments
We thank Dan Draganovici and Ewa Radomska for expert technical support.
Footnotes
Address reprint requests to PD Dr. Hans-Joachim Anders, Medizinische Poliklinik, Klinikum der Universität München–Innenstadt, Pettenkoferstr. 8a, 80336 Munchen, Germany. E-mail: hjanders@med.uni-muenchen.de.
Supported by the Else-Kroener-Fresenius Foundation (grant 80736030 to H.-J.A.; grant to S.S.) and the European Union (Network of Excellence MAIN FP6-502935 to H.-J.A., D.S., and F.K. and Integrated Project INNOCHEM FP6-518167 to H.-J.A. and P.J.N.).
Parts of this project were prepared by V.N. as a doctoral thesis at the Faculty of Medicine, University of Munich, Munich, Germany.
References
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li Q, Li S, Qiu Y, Li S, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zhang R, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L. United States renal data system 2006 annual data report. Am J Kidney Dis. 2007;49(1 Suppl 1):A6–A7. doi: 10.1053/j.ajkd.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Svensson M, Sundkvist G, Arnqvist HJ, Bjork E, Blohme G, Bolinder J, Henricsson M, Nystrom L, Torffvit O, Waernbaum I, Ostman J, Eriksson JW. Diabetes Incidence Study in Sweden (DISS): signs of nephropathy may occur early in young adults with diabetes despite modern diabetes management: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS). Diabetes Care. 2003;26:2903–2909. doi: 10.2337/diacare.26.10.2903. [DOI] [PubMed] [Google Scholar]
- Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005;16(Suppl 1):S30–S33. doi: 10.1681/asn.2004110970. [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17:368–377. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- Mora C, Navarro JF. The role of inflammation as a pathogenic factor in the development of renal disease in diabetes. Curr Diab Rep. 2005;5:399–401. doi: 10.1007/s11892-005-0044-x. [DOI] [PubMed] [Google Scholar]
- Meyer TW. Immunosuppression for diabetic glomerular disease? Kidney Int. 2003;63:377–378. doi: 10.1046/j.1523-1755.2003.00747.x. [DOI] [PubMed] [Google Scholar]
- Tuttle KR. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol. 2005;16:1537–1538. doi: 10.1681/ASN.2005040393. [DOI] [PubMed] [Google Scholar]
- Sassy-Prigent C, Heudes D, Mandet C, Belair MF, Michel O, Perdereau B, Bariety J, Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–475. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- Yozai K, Shikata K, Sasaki M, Tone A, Ohga S, Usui H, Okada S, Wada J, Nagase R, Ogawa D, Shikata Y, Makino H. Methotrexate prevents renal injury in experimental diabetic rats via anti-inflammatory actions. J Am Soc Nephrol. 2005;16:3326–3338. doi: 10.1681/ASN.2004111011. [DOI] [PubMed] [Google Scholar]
- Utimura R, Fujihara CK, Mattar AL, Malheiros DM, Noronha IL, Zatz R. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 2003;63:209–216. doi: 10.1046/j.1523-1755.2003.00736.x. [DOI] [PubMed] [Google Scholar]
- Dalla Vestra M, Mussap M, Gallina P, Bruseghin M, Cernigoi AM, Saller A, Plebani M, Fioretto P. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(Suppl 1):S78–S82. doi: 10.1681/asn.2004110961. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42:53–61. doi: 10.1016/s0272-6386(03)00408-6. [DOI] [PubMed] [Google Scholar]
- Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, Yoshioka N, Imai H, Kakei M, Ito S. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications. 2003;17:11–15. doi: 10.1016/s1056-8727(02)00176-9. [DOI] [PubMed] [Google Scholar]
- Bohle A, Wehrmann M, Bogenschutz O, Batz C, Muller CA, Muller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187:251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- Furuta T, Saito T, Ootaka T, Soma J, Obara K, Abe K, Yoshinaga K. The role of macrophages in diabetic glomerulosclerosis. Am J Kidney Dis. 1993;21:480–485. doi: 10.1016/s0272-6386(12)80393-3. [DOI] [PubMed] [Google Scholar]
- Benigni A, Zoja C, Corna D, Zatelli C, Conti S, Campana M, Gagliardini E, Rottoli D, Zanchi C, Abbate M, Ledbetter S, Remuzzi G. Add-on anti-TGF-beta antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol. 2003;14:1816–1824. doi: 10.1097/01.asn.0000074238.61967.b7. [DOI] [PubMed] [Google Scholar]
- Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Chanty A, Gow RM, Zhang Y, Gilbert RE. Protein kinase Cbeta inhibition attenuates osteopontin expression, macrophage recruitment, and tubulointerstitial injury in advanced experimental diabetic nephropathy. J Am Soc Nephrol. 2005;16:1654–1660. doi: 10.1681/ASN.2004070578. [DOI] [PubMed] [Google Scholar]
- Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Hara A, Yamahana J, Okumura T, Takasawa K, Takeda S, Yoshimura M, Kida H, Yokoyama H. Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am J Kidney Dis. 2005;45:54–65. doi: 10.1053/j.ajkd.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Wilson HM, Walbaum D, Rees AJ. Macrophages and the kidney. Curr Opin Nephrol Hypertens. 2004;13:285–290. doi: 10.1097/00041552-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Sean Eardley K, Cockwell P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005;68:437–455. doi: 10.1111/j.1523-1755.2005.00422.x. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol. 2005;16:1711–1722. doi: 10.1681/ASN.2004070612. [DOI] [PubMed] [Google Scholar]
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Ninichuk V, Schlondorff D. Progression of kidney disease: blocking leukocyte recruitment with chemokine receptor CCR-1 antagonists. Kidney Int. 2006;69:29–32. doi: 10.1038/sj.ki.5000053. [DOI] [PubMed] [Google Scholar]
- Ninichuk V, Gross O, Reichel C, Khandoga A, Pawar RD, Ciubar R, Segerer S, Belemezova E, Radomska E, Luckow B, de Lema GP, Murphy PM, Gao JL, Henger A, Kretzler M, Horuk R, Weber M, Krombach F, Schlondorff D, Anders HJ. Delayed chemokine receptor 1 blockade prolongs survival in collagen 4A3-deficient mice with Alport disease. J Am Soc Nephrol. 2005;16:977–985. doi: 10.1681/ASN.2004100871. [DOI] [PubMed] [Google Scholar]
- Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- Bower G, Brown DM, Steffes MW, Vernier RL, Mauer SM. Studies of the glomerular mesangium and the juxtaglomerular apparatus in the genetically diabetic mouse. Lab Invest. 1980;43:333–341. [PubMed] [Google Scholar]
- Revesz L, Bollbuck B, Buhl T, Dawson J, Feifel R, Heng R, Hiestand P, Sparrer H, Schlapbach A, Waelchli R, Loetscher P. Bridged piperazines and piperidines as CCR1 antagonists with oral activity in models of arthritis and multiple sclerosis. Lett Drug Design Discov. 2006;3:689–694. [Google Scholar]
- Haverty TP, Kelly CJ, Hines WH, Amenta PS, Watanabe M, Harper RA, Kefalides NA, Neilson EG. Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol. 1988;107:1359–1368. doi: 10.1083/jcb.107.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders HJ, Belemezova E, Eis V, Segerer S, Vielhauer V, Perez de, Lema G, Kretzler M, Cohen CD, Frink M, Horuk R, Hudkins KL, Alpers CE, Mampaso F, Schlondorff D. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J Am Soc Nephrol. 2004;15:1504–1513. doi: 10.1097/01.asn.0000130082.67775.60. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Vielhauer V, Schlondorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63:401–415. doi: 10.1046/j.1523-1755.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Bader R, Bader H, Grund KE, Mackensen-Haen S, Christ H, Bohle A. Structure and function of the kidney in diabetic glomerulosclerosis. Correlations between morphological and functional parameters. Pathol Res Pract. 1980;167:204–216. doi: 10.1016/S0344-0338(80)80051-3. [DOI] [PubMed] [Google Scholar]
- William J, Hogan D, Batlle D. Predicting the development of diabetic nephropathy and its progression. Adv Chronic Kidney Dis. 2005;12:202–211. doi: 10.1053/j.ackd.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Erwig LP, Kluth DC, Rees AJ. Macrophage heterogeneity in renal inflammation. Nephrol Dial Transplant. 2003;18:1962–1965. doi: 10.1093/ndt/gfg313. [DOI] [PubMed] [Google Scholar]
- Haberstroh U, Pocock J, Gomez-Guerrero C, Helmchen U, Hamann A, Gutierrez-Ramos JC, Stahl RA, Thaiss F. Expression of the chemokines MCP-1/CCL2 and RANTES/CCL5 is differentially regulated by infiltrating inflammatory cells. Kidney Int. 2002;62:1264–1276. doi: 10.1111/j.1523-1755.2002.kid572.x. [DOI] [PubMed] [Google Scholar]
- Luster AD. Chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28:2686–2690. doi: 10.2337/diacare.28.11.2686. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Anderson S, Gordon KL, Oyama TT, Shankland SJ, Johnson RJ. Tubulointerstitial disease in aging: evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol. 1998;9:231–242. doi: 10.1681/ASN.V92231. [DOI] [PubMed] [Google Scholar]