Abstract
Intracellular protein deposition due to aggregation caused by conformational alteration is the hallmark of a number of neurodegenerative disorders, including Parkinson’s disease, tauopathies, Huntington’s disease, and familial encephalopathy with neuroserpin inclusion bodies. The latter is an autosomal dominant disorder caused by point mutations in neuroserpin resulting in its destabilization. Mutant neuroserpin polymerizes and forms intracellular aggregates that eventually lead to neurodegeneration. We generated genetically modified mice expressing the late-onset S49P-Syracuse or the early-onset S52R-Portland mutation of neuroserpin in central nervous system neurons. Mice exhibited morphological, biochemical, and clinical features resembling those found in the human disease. Analysis of brains revealed large intraneuronal inclusions composed exclusively of mutant neuroserpin, accumulating long before the development of clinical symptoms in a time-dependent manner. Clinical symptoms and amount of neuroserpin inclusions correlated with the predicted instability of the protein. The presence of inclusion bodies in subclinical mice indicates that in humans the prevalence of the disease could be higher than anticipated. In addition to shedding light on the pathophysiology of the human disorder, these mice provide an excellent model to study mechanisms of neurodegeneration or establish novel therapies for familial encephalopathy with neuroserpin inclusion bodies and other neurodegenerative diseases with intracellular protein deposition.
Aggregation and tissue deposition of specific proteins is thought to play an important role in the pathogenesis of many disorders referred to as conformational diseases, including Alzheimer’s disease, Huntington’s disease, prion diseases, and familial Parkinson’s disease.1 The serpinopathies represent a group of conformational diseases characterized by aggregation and tissue deposition of proteins belonging to the serpin superfamily of serine protease inhibitors.
Serpins inhibit their target proteases through covalent interaction. The inhibitory mechanism starts with the formation of a complex between the active site of the protease and the reactive site loop of the inhibitor that represents a pseudosubstrate for the target protease. Complex formation is followed by cleavage of the reactive site loop of the serpin and by insertion of the N-terminal segment of the cleaved reactive site loop into β-sheet A of the serpin. As a consequence, the protease is translocated to the opposite pole of the serpin, distorted, and thus inactivated by a mousetrap action.2,3 This inhibitory mechanism has the advantage to ensure an effective irreversible inhibition. However, the ability to accept the reactive site loop as an additional β-strand renders the serpins vulnerable to the formation of intermolecular associations. Such intermolecular insertions result in intracellular protein aggregation and eventually lead to pathological conditions called serpinopathies.4,5
Serpinopathies are caused by mutations that destabilize the β-sheet A and allow the incorporation of the reactive site loop of another molecule as an additional β-strand, leading to the formation of polymers that are retained within the cells. This process is best characterized in mutants of α1-antitrypsin that result in accumulation of the protein as inclusions in the rough endoplasmic reticulum (ER) of the liver, thereby leading to damage in the form of juvenile hepatitis, liver cirrhosis, and hepatocellular carcinoma.5
Familial encephalopathy with neuroserpin inclusion bodies (FENIB) is linked to autosomal dominantly inherited mutations in the gene encoding neuroserpin, a serine protease inhibitor expressed in neurons of the central and peripheral nervous system.6,7 Four mutations have been identified. They are located in the shutter region of neuroserpin that controls the shutter-like opening and closure of the β-sheet A and thereby regulates the insertion of the reactive site loop into the β-sheet A.8,9 The mutations are responsible for the generation of polymers of neuroserpin molecules that accumulate in the brain and lead to neuronal death. Histological analysis of brain sections revealed the presence of eosinophilic inclusion bodies (called Collins bodies) throughout the deeper layers of the cerebral cortex and in many subcortical nuclei. Biochemical analysis revealed that the periodic acid-Schiff (PAS)-positive, diastase-resistant inclusion bodies consist exclusively of neuroserpin. The four mutations lead to varying degree of instability within neuroserpin. As a consequence, patients carrying a mutation responsible for a high instability have an earlier disease onset, more severe clinical manifestations, a shorter life expectancy, and more inclusion bodies when compared with patients with more stable forms of neuroserpin.
Although analyses of brain material from FENIB patients have provided valuable insights into the terminal stage of the disease, essential questions regarding the early phase of the neurodegeneration remain unanswered to date. Furthermore, it remains enigmatic whether the generation of neuroserpin inclusions invariably leads to the development of clinical disease. The fact that all FENIB patients were shown to harbor abundant deposits of mutant neuroserpin does not necessarily suggest that a subclinical carrier state for FENIB does not exist. If one deems a subclinical carrier state for FENIB possible, the implications for the prevalence of the disease are obvious.
We have generated genetically modified mice overexpressing mutant forms of human neuroserpin (S49P-Syracuse and S52R-Portland mutations). Mice expressing mutant neuroserpin showed clinical symptoms and inclusion body accumulation reminiscent of those seen in FENIB patients. Immunohistochemical and Western blot analysis of the inclusions confirmed the exclusive presence of mutant neuroserpin, whereas electron microscopic analysis revealed the localization of the aggregates in the ER. No inclusion bodies were detected in control mice overexpressing wild-type neuroserpin at any stage. Mice expressing the unstable Portland neuroserpin displayed more severe clinical symptoms and harbored more neuroserpin deposits than Syracuse mice, thus confirming the hypothesis that the structural instability of neuroserpin correlates with the severity of the disease. Interestingly, accumulation of mutant neuroserpin preceded development of clinical symptoms, leading to a subclinical disease.
This study provides valuable insights into the pathophysiology of FENIB. We believe that our transgenic model will be invaluable in determining processes of neurodegeneration and in establishing possible therapeutic options in conformational diseases.
Materials and Methods
Anti-Neuroserpin Antibody
The polyclonal anti-neuroserpin antibody was generated by immunizing a goat with recombinant mouse neuroserpin (amino acids 17 to 397) purified by anion exchange and hydrophobic interaction chromatography as described previously.7 The antibody was affinity-purified with the same recombinant neuroserpin that was used for immunization.
Generation of Transgenic Mice
S49P-Syracuse and S52R-Portland mutations were introduced into the human neuroserpin cDNA using the polymerase chain reaction technique. The three 1400-bp cDNA fragments of wild-type, Syracuse, and Portland neuroserpin were inserted into a Thy-1 vector.10 The fragments containing the cDNA of interest were excised with PvuI and purified from a 1% agarose gel with the QIAEXII extraction kit (Qiagen, Basel, Switzerland). Pronuclear injections into fertilized oocytes were performed by standard methods. Transgenic mice were identified by polymerase chain reaction using the following primers: 5′-TCCCCACCACAGAATCCAAGTCG-3′ hybridizing to exon 2 of Thy-1 and 5′-CACCCACTTATTGATGTAGTTGG-3′ hybridizing to human neuroserpin. As a positive control for polymerase chain reaction, a fragment of the TAG-1 gene was amplified with the following primers: 5′-ACACGAAGTGACGCCCATCCGT-3′ and 5′-GGAGGAGAGAGACCCCGTGAAA-3′. The analyzed transgenic lines were generated on a B6D2F2 hybrid background and backcrossed to C57BL/6 for at least five generations. All animal experiments were performed in accordance with the guidelines and regulations of the Swiss veterinary authority.
Quantification of Neuroserpin Protein Levels
Brain homogenates were prepared as follows. Brains were dissected from adult animals and homogenized in a buffer containing 5 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 320 mmol/L sucrose, pH 7.4, using a Potter-Elvehjem homogenizer. Nuclei were removed by short centrifugation, and proteins were solubilized by the addition of 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate to a final concentration of 1%. Extracts were cleared from insoluble material by ultracentrifugation, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and blotted onto nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). Because our mouse lines express varying amounts of the transgene, different amounts of extract were loaded, to allow the detection of bands in the same intensity range, thus avoiding saturation of some signals. For immunodetection, an affinity-purified goat anti-neuroserpin antibody was used (2 μg/ml), followed by horseradish peroxidase-conjugated rabbit anti-goat IgG (Sigma-Aldrich, Buchs, Switzerland), and visualized with FluorChem 8000 (Alpha Innotech Corporation, San Leandro, CA). Densitometric quantification was performed using the spot density function of FluorChem 8000.
Histological Analysis
Mice were perfused with 4% paraformaldehyde in phosphate-buffered saline (pH 7.5). Brains were dissected and fixed for 16 hours in 4% paraformaldehyde in phosphate-buffered saline (pH 7.5), paraffin-embedded, and cut into 2- to 3-μm sections. Sections were stained with PAS according to routine methods.
For immunohistochemical staining, paraffin sections were deparaffinized, hydrated, and boiled in a sodium citrate buffer to retrieve the antigen. The affinity-purified goat anti-neuroserpin antibody was used at 10 μg/ml. Immunoreactivity was visualized with a biotin-conjugated secondary antibody using the avidin-biotin-complex technique and diaminobenzidine as a chromogen (basic DAB kit; Ventana, Illkirch, France). For quantification of inclusion bodies, three regions of hippocampus (subiculum, CA1, and CA3) and three regions of cerebral cortex of one hemisphere were assessed. The immunoreactive area was quantified as percentage of the total area for each image using the AnalySIS 3.0 program (Soft Imaging System GmbH, Münster, Germany), and the average of six images was calculated. Two animals per line and time point were analyzed.
For electron microscopy, brain tissue was postfixed with 3% glutaraldehyde and embedded in epoxy resin.
Isolation of Inclusion Bodies
Two mouse brains from animals overexpressing wild-type (lines 682, 14 months old) and Portland neuroserpin (line 794, 16 months old) were analyzed. The isolation was performed following the protocol described by Davis et al11 with some modifications. Collagenase Liberase Blendzyme 3 (Roche Diagnostics, Rotkreuz, Switzerland) was used in the collagenase digestion step together with 100 μl of DNaseI (10 mg/ml; Roche Diagnostics). After centrifugation at 45,000 × g for 60 minutes, the pellet was resuspended in 2 ml of homogenization medium and passed several times through a syringe needle to reduce viscosity and centrifuged again. The resulting pellet was resuspended in 100 μl of 4% SDS, 20% glycerol, 10% mercaptoethanol, 125 mmol/L Tris-HCl, pH 6.8, and heated for 2 hours at 75°C. After the last centrifugation step at 14,000 × g for 10 minutes, the supernatant was analyzed by SDS-PAGE followed by Coomassie blue staining or Western blot analysis with the affinity-purified goat anti-neuroserpin antibody.
Protein Sequence Analysis
Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane by electroblotting. The membrane was stained with Coomassie blue. The desired band was cut, and Edman degradation was performed by the Protein Analysis Group of the Institute of Biochemistry, University of Zurich, on a model G1005A protein sequencer (Agilent Technologies, Palo Alto, CA) according to the manufacturer’s recommendations.
Protein Identification
After SDS-PAGE and Coomassie blue staining, the protein band was cut out and protein identification was performed by the Protein Analysis Group of the Institute of Biochemistry, University of Zurich, using a QTOF mass spectrometer (Waters, Milford, MA) equipped with a capillary high-performance liquid chromatography (Waters). In-gel tryptic digestion was performed according to published protocols.12,13 In brief, the gel band was cut into small pieces. Gel pieces were washed twice with 100 mmol/L ammonium bicarbonate/50% (v/v) acetonitrile for 30 minutes at 30°C and once with acetonitrile. All supernatants were discarded. The gel pieces were rehydrated with trypsin solution and incubated in digestion buffer at 37°C overnight. Afterward, the supernatant was removed, and peptides were extracted twice with 0.1% (v/v) trifluoroacetic acid/50% (v/v) acetonitrile. The combined supernatants were dried and dissolved in 0.1% (v/v) formic acid for analysis. Database search was performed using the Mascot search engine.14
Subcellular Fractionation
Four brains were dissected from animals older than 22 months overexpressing wild-type or Syracuse neuroserpin. Tissue was homogenized in 4 ml of buffer (130 mmol/L KCl, 25 mmol/L NaCl, 1 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, and 25 mmol/L Tris-HCl, pH 7.4) and centrifuged for 10 minutes at 1000 × g. The supernatant was removed, centrifuged for 10 minutes at 3000 × g, and layered on a step gradient consisting of 1 ml each of 30, 25, 20, 15, 12.5, 10, 7.5, 5, and 2.5% (v/v) iodixanol (OptiPrep density gradient medium; Sigma-Aldrich) in homogenization buffer. The gradient was centrifuged at 126,000 × g for 1 hour. Eleven fractions were collected from the top of the gradient and analyzed by Western blot analysis with the affinity-purified goat anti-neuroserpin antibody, the polyclonal anti-calnexin antibody C-20 (Santa Cruz Biotechnology, Heidelberg, Germany), and the monoclonal anti-β-COP antibody maD (Sigma-Aldrich).
Results
Overexpression of Wild-Type and Mutant Neuroserpin in Genetically Modified Mice
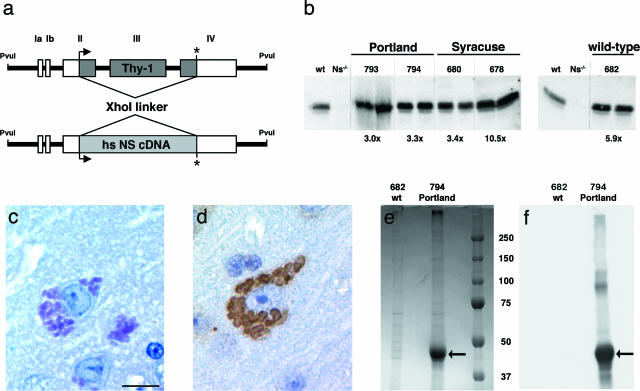
Transgenic mice overexpressing wild-type, S49P-Syracuse, and S52R-Portland human neuroserpin under the control of the Thy-1 promoter were generated using an expression cassette derived from the murine Thy-1 gene (Figure 1a).10 As expected, the transgene was expressed in central nervous system neurons throughout postnatal life. We quantified the levels of neuroserpin in brain homogenates by Western blotting using an affinity-purified anti-neuroserpin antibody. For negative control, brain homogenate from a neuroserpin-deficient mouse15 was used. The expression levels for transgenic mice overexpressing human Syracuse neuroserpin were 3.4 [line Tg(NSS49P)680Zbz] and 10.5 times [line Tg(NSS49P)678Zbz], for transgenic mice with human Portland neuroserpin 3.0 [line Tg(NSS52R)793Zbz] and 3.3 times [line Tg(NSS52R)794Zbz], and for transgenic mice with human wild-type neuroserpin 5.9 times [line Tg(NS)682Zbz] above the endogenous neuroserpin level of wild-type mice (Figure 1b).
Figure 1.
Generation and characterization of transgenic mice overexpressing human Portland, Syracuse, and wild-type neuroserpin. a: Schematic drawing of the DNA construct used to generate transgenic mice. The translated region of Thy-1 was replaced by neuroserpin cDNA. Relevant restriction sites are indicated. The PvuI-PvuI fragment was injected into the pronucleus of fertilized oocytes. b: Quantification of neuroserpin protein level by Western blotting of brain homogenates of mice overexpressing Portland (lines 793 and 794), Syracuse (lines 678 and 680), and wild-type neuroserpin (line 682). Two animals per line were analyzed. As a negative control, brain homogenate from a neuroserpin-deficient mutant (Ns−/−) mouse was loaded. The degree of overexpression, determined by comparing band intensities with a nontransgenic C57BL/6 mouse (wt), is indicated below each panel. PAS staining (c) and neuroserpin immunohistochemistry (d) of brain sections of a Portland transgenic mouse (line 793) aged 6 months reveals the accumulation of neuroserpin inclusions identical to those observed in FENIB patients. Coomassie blue-stained SDS-PAGE gel (e) and Western blot (f) of inclusion bodies isolated from brains of transgenic mice overexpressing Portland neuroserpin (line 794). A single band is present (arrow); it reacts positively with an affinity-purified goat antibody against neuroserpin. Molecular mass marker applies for both pictures (e and f). Scale bar in c = 100 μm.
Clinical Symptoms Reminiscent of Those Observed in FENIB Patients in Mutant Neuroserpin Mice
Transgenic mice appeared healthy at birth, developed normally, and did not exhibit any obvious neurological or behavioral abnormalities during early adulthood. However, at 17 and 21 months of age for the Portland lines 794 and 793, respectively, and at 23 months for the Syracuse line 678, mice began to exhibit severe neurological symptoms and behavioral abnormalities, reminiscent of those found in FENIB patients (see Supplementary Video 1, at http://ajp.amjpathol.org). They developed severe ataxia, moved slowly with staggering gait, were incapable of grasping a horizontal wire and climbing a grid of negative geotaxis, and exhibited a considerably greater passivity during handling. In addition, mice overexpressing Portland neuroserpin showed tremor and seizure-like episodes. On appearance of clinical symptoms, the mice were euthanized. Mice of Syracuse line 680, as well as mice overexpressing wild-type neuroserpin, did not show clinical symptoms up to 27 months of age.
Mice Overexpressing Mutant Neuroserpin Show Intraneuronal Inclusion Bodies
Postmortem inspection of mice did not reveal any macroscopic abnormalities in investigated organs (brain, spinal cord, liver, kidney, lung). Histological examination of the central nervous system of all mice overexpressing Syracuse and Portland neuroserpin showed massive intraneuronal PAS-positive depositions of round bodies reminiscent of the inclusions observed in brain sections of FENIB patients.8 Affected cells showed one or more inclusions, sometimes numerous and arranged in grape-like clusters (Figure 1c). The presence of inclusion bodies was accompanied by a loss of neurons in hippocampus and cerebral cortex. Glial cells seemed to be spared from these changes. Age-matched mice overexpressing human wild-type neuroserpin (line 682) did not exhibit intraneuronal inclusion bodies.
Inclusion Bodies Exclusively Consist of Mutant Neuroserpin
All intracellular inclusions exhibited strong neuroserpin immunoreactivity (Figure 1d). In accordance with observations made in brain sections of FENIB patients, the inclusions showed strongest staining at the periphery.16 To analyze their molecular composition, we isolated inclusion bodies from genetically modified mice overexpressing Portland neuroserpin (n = 2, line 794) and from mice overexpressing wild-type neuroserpin (n = 2, line 682) according to published protocols.8,11 Coomassie blue staining revealed a single strong band of approximately 46 kd in the sample obtained from mice overexpressing mutant neuroserpin, which was absent in the control sample (Figure 1e). The band exhibited strong neuroserpin immunoreactivity on the Western blot probed with an affinity-purified anti-neuroserpin antibody (Figure 1f). Amino terminal sequencing by Edman degradation revealed the sequence ATFPE corresponding to the amino terminus of mature human neuroserpin starting at amino acid 19 and thus confirmed the mutant nature of the neuroserpin accumulated in the inclusion bodies. Digestion by trypsin followed by liquid chromatography and tandem mass spectrometric analysis revealed 11 peptides. All corresponded to tryptic peptide fragments of human neuroserpin and together covered 23.7% of the sequence. Seven were unique peptides of human neuroserpin, and three of them were observed two or more times. No peptides specific for murine neuroserpin were found. Moreover, a Portland neuroserpin-specific tryptic peptide was identified. This was generated by cleavage after arginine 52, which is the amino acid that is replaced in the mutant protein. These results demonstrate that Collins bodies are exclusively made up of mutant neuroserpin.
Preclinical Appearance of Neuroserpin Inclusion Bodies
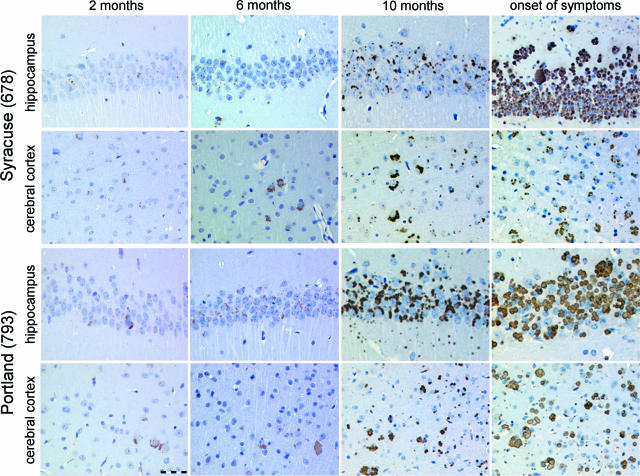
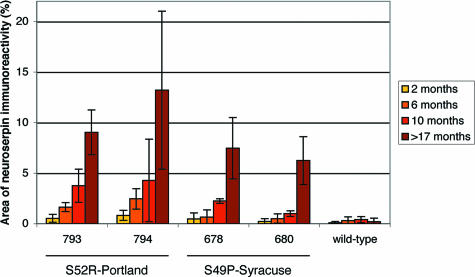
To follow the dynamic evolution of inclusion body formation, we euthanized mice at 2, 6, and 10 months of age, as well as at the onset of clinical symptoms (17 and 21 months for Portland lines 794 and 793, respectively, and 23 months for Syracuse line 678). Mice of the Syracuse line 680 and of wild-type line 682, which did not develop clinical symptoms, were euthanized at 21 months of age. Already at 2 months of age, the animals showed occasional inclusions (Figure 2). The number of inclusions increased with age. In general, inclusions were markedly more frequent and larger in mice expressing the Portland mutation. We quantified inclusion bodies in our mouse models as previously described.17 A graphical representation of the results is shown in Figure 3. During the early stages of inclusion body formation, from the second to the tenth month of life, the area of neuroserpin-immunoreactive inclusions increased exponentially with time. At every stage, the mice of the Portland lines overexpressing the less stable S52R mutant of neuroserpin showed more inclusions than those of the Syracuse lines. Even a threefold higher concentration of Syracuse neuroserpin, as found in line 678, was not able to compensate for the difference. One line of mice expressing Syracuse neuroserpin (line 680), which did not show clinical symptoms up to 27 months of age, was shown to accumulate massive amounts of neuroserpin inclusion bodies.
Figure 2.
Time course of neuroserpin accumulation. Formalin-fixed, paraffin-embedded sections of the CA1 region of the hippocampus and the cerebral cortex of transgenic mice overexpressing Portland (line 793) and Syracuse (line 678) neuroserpin were stained with an affinity-purified goat polyclonal antibody against neuroserpin to visualize the inclusion bodies. Scale bar = 50 μm.
Figure 3.
Quantification of inclusion body deposition in brains of transgenic mice overexpressing Portland (lines 793 and 794), Syracuse (lines 678 and 680), and wild-type neuroserpin (line 682). The area of the inclusions was quantified at four time points and presented as percentage of the total area. The values represent the average of six areas (three cerebral cortex and three hippocampal regions) in two animals.
Neuroserpin Inclusion Bodies Are Localized within the Rough ER
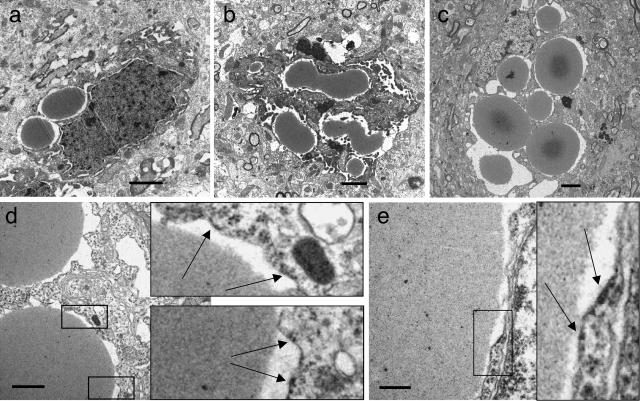
The temporal development of the inclusion bodies was confirmed by ultrastructural studies (Figure 4, a–c). With mice of line 793, we found inclusions of about 2 to 3 μm in diameter at 6 months of age, with most cells having only one or two inclusion bodies. At 10 months of age, inclusions were bigger and more numerous. Their shape was sometimes oval or irregular rather than round, suggesting that they had resulted from fusions between two or more inclusion bodies. At a very late stage, around 21 months of age, affected cells were filled with inclusions of different shape and size, many having a homogenous appearance with little internal structure, others showing a dark core. The nuclei of the affected cells were usually eccentric, often deformed and compressed. The cytoplasm and inner organelles were hardly recognizable. Electron microscopic analysis also demonstrated that most inclusions were surrounded by a membrane bearing occasional ribosomes, suggesting that the Collins bodies are retained within the rough ER (Figure 4, d–e).
Figure 4.
Ultrastructural analysis of brains of Portland neuroserpin mice (line 793). At 6 months (a), inclusion bodies are round or oval. At 10 months (b), they are more numerous, larger, and have a more complex shape, suggesting that they may be the product of fusions. At late time points (c, 21 months), inclusions fill the entire cell, thus deforming it. In d and e, arrows indicate occasional ribosomes on the membrane surrounding inclusions in the brain of a 21-month-old Portland neuroserpin transgenic mouse. Scale bars: a–c, 2 μm; d, 1 μm; e, 0.3 μm.
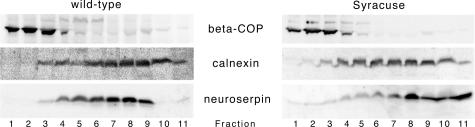
To analyze further the subcellular localization of the inclusion bodies, we fractionated Golgi and ER compartments of 22-month-old mice overexpressing Syracuse neuroserpin and age-matched mice overexpressing wild-type neuroserpin in a discontinuous iodixanol gradient by sedimentation velocity. Western blot analysis revealed an accumulation of neuroserpin in the endoplasmic reticulum for both mouse groups (Figure 5). However, the mutant protein was predominantly retained within the denser, heavier fractions at the bottom of the gradient, and no signal was found in the Golgi compartment.
Figure 5.
Subcellular localization of neuroserpin in brains of transgenic mice overexpressing wild-type and Syracuse neuroserpin. Western blot analysis of fractions obtained by separating ER and Golgi compartments using discontinuous iodixanol gradients. Fractions were collected from top of the gradients. Marker proteins are calnexin (ER) and β-COP (Golgi).
Discussion
FENIB is a conformational dementia caused by mutations in the gene encoding neuroserpin and is inherited in an autosomal dominant fashion. The mutations are located in the shutter region of neuroserpin and cause a premature, target protease-independent insertion of the reactive site loop into the β-sheet A of another molecule. The conformational effect of the different mutations varies considerably, and so does the age of onset of clinical symptoms in affected patients. The domain controlling the opening of the main sheet of neuroserpin is centered on Ser49; however, Ser52 forms an even more critical interaction with the sheet.8 As a consequence, late-onset clinical symptoms were found in patients with the S49P-Syracuse mutation, early-onset clinical symptoms were found in patients with the S52R-Portland, and subsequently with the H338R and the G392E mutations. Although some of the molecular prerequisites for neuroserpin polymer formation are known, our knowledge on the pathophysiology of FENIB is limited, and to date there are no therapeutic protocols for FENIB. Here, we present data on the generation and the analysis of genetically modified mice expressing late-onset Syracuse and early-onset Portland mutations of neuroserpin.
Genetically modified mice were generated by pronuclear injection of constructs containing wild-type or mutant neuroserpin cDNA. The transgenes were expressed under the regulatory sequences of the Thy-1 promoter. The latter was previously shown to be active in central nervous system neurons throughout postnatal life.10 Two mouse lines per each mutation were analyzed, each overexpressing different amounts of the proteins, to investigate the effect of protein amount on the accumulation of inclusion bodies. As a control, we used transgenic mice overexpressing wild-type human neuroserpin instead of wild-type littermates, to exclude the possibility that inclusion bodies accumulate due to overexpression of the protein, as reported for another serpin, megsin.18,19 A total of four mouse lines expressing mutant neuroserpin were investigated; therefore, we can exclude that the phenotype is an effect of the site of integration of the transgene.
At different time points, mice of both Portland lines and mice of Syracuse line 678 exhibited a phenotype reminiscent of that found in FENIB patients,20 including severe ataxia, slow movement, and, for Portland mice, tremor and seizure-like episodes. Interestingly, the latter symptoms have also been reported for patients harboring the Portland but not the Syracuse mutation. In a previous study,21 it was speculated that lack of inhibitory activity of Portland neuroserpin contributed to symptoms like epileptic seizures in FENIB. However, neuroserpin-deficient mice have been generated.15 They are viable, healthy, and, in contrast to the Portland mice, do not show seizure-like episodes, even in later stages of life. Therefore, we think that inactivity of neuroserpin alone is not sufficient to cause symptoms like epileptic seizure.
Mice overexpressing Portland and Syracuse mutations accumulate intracellular PAS-positive inclusion bodies in neurons that react positively with anti-neuroserpin antibody. The appearance of the inclusions was very similar to that reported previously for FENIB patients.11,16 We also observed one or more inclusions per cell, accumulating in a grape-like structure, crowding aside other cytoplasmic organelles. Immunohistochemistry showed strongest staining at the periphery, and electron microscopic analysis often showed a dark core. Moreover, as previously described,11,16,22 the localization of the inclusions within the membranes of the rough ER could be demonstrated in our mouse models by ultrastructural analysis and subcellular fractionation. Examination of inclusion bodies isolated from the brains of our mice confirmed that they are composed of a single protein, neuroserpin. Besides the thick 46-kd band of monomeric neuroserpin, a band of approximately 100 kd as well as a band on top of the blot also reacted with the anti-neuroserpin antibody. They probably represent dimers and polymers of neuroserpin that could not be separated. We could isolate only the human protein from inclusion bodies, because N-terminal sequencing of the isolated protein revealed ATFPE, which corresponds to the N terminus of human neuroserpin, and not ATFPD, the N terminus of the mouse sequence. Therefore, as already shown for the human disease,23 no endogenous, wild-type neuroserpin was found within inclusion bodies, confirming that synthesis and processing of normal neuroserpin is unaltered. N-terminal sequencing of the isolated protein revealed that the accumulated neuroserpin is secreted past the ribosomal membrane with cleavage of the signal peptide. However, the N terminus of the accumulated neuroserpin, starting at amino acid 19, does not correspond to the proposed signal peptidase cleavage site,24 which is located between amino acids 16 and 17. The same discrepancy has already been observed for neuroserpin isolated from inclusion bodies of human patients.11,23
The accumulation of inclusion bodies in the brains of the transgenic mice was studied at different time points and compared between Portland and Syracuse mice. When comparing mouse lines overexpressing the same mutation of neuroserpin, the increase in accumulated inclusion bodies not only correlated with time but was also dependent on the expression level, because mice overexpressing more protein accumulated more inclusion bodies in the brain. This effect has already been described for the accumulation of another serpin, α1-antitrypsin.25 However, at any stage, Portland mice accumulated more inclusions compared with Syracuse mice. This effect could not be compensated by a higher concentration of Syracuse neuroserpin, as found in line 678, and is in accordance with a considerably earlier onset of disease and more severe symptoms observed in our mice as well as in humans affected with this mutation. Thus, by comparing syngeneic mice that differ in only one mutation in neuroserpin, we could confirm that the clinical manifestations, the amount of inclusions, and the severity of the disease correlate with the predicted instability of neuroserpin rather than with the expression level. Moreover, the rates of accumulation of mutant neuroserpin in our mice reflect those measured in vitro for recombinantly expressed Syracuse and Portland neuroserpin.21,26 Both proteins rapidly formed polymers at 37°C as well as at 45°C, with Portland neuroserpin presenting faster rates of polymerization compared with the Syracuse variant.
Previous knowledge about FENIB was based on observations made on material acquired from patients after the onset of clinical symptoms. Our mouse models offer the possibility of studying the course of the disease before the onset of symptoms. The most important finding of this study is that we were able to observe deposits of mutant neuroserpin starting from the age of 2 months, long before the onset of the disease. When clinical symptoms appear, most of the neurons are already filled with inclusion bodies. The presence of a preclinical disease state has been described for a number of dementias.27 The fact that this has now been shown for FENIB supports the theory that the central nervous system is able to compensate for neuronal loss to a certain degree. Once a critical level of neuronal loss is exceeded, clinical symptoms invariably occur. On the other hand, mice of Syracuse line 678 show a phenotype at 23 months of age, whereas mice of line 680 (overexpressing less Syracuse neuroserpin compared with 678) show inclusion body accumulation but do not develop clinical manifestations during their life span. One could speculate that these mice would eventually develop the disease if they would live longer. For this reason, we speak of a subclinical disease. A subclinical state despite significant inclusion body formation may imply the presence of subclinically affected FENIB individuals, signifying that FENIB could be more common than previously thought.
Recently, transgenic rats overexpressing another serpin, megsin, were described as a model for FENIB.19 Heterozygous rats show a slowly progressive neurodegeneration with accumulation of PAS-positive, diastase-resistant inclusion bodies in neurons. Neuronal loss has been reported, but the animal have a similar life span compared with nontransgenic rats. Although megsin transgenic rats may represent an interesting model, we think that genetically modified animals overexpressing different mutations of the same protein that accumulate in FENIB under the control of a neuron-specific promoter represent a better tool for studies aimed at understanding the cellular response to intracellular protein aggregation in neuronal tissue, for FENIB as well as for other neurodegenerative diseases. A model where mutant neuroserpin is expressed at physiological level under transcriptional control of its own promoter would be desirable. However, it is conceivable that these mice would not develop disease during their natural lifespan due to the low expression level of the mutant protein. Therefore, we believe that our model will be an indispensable tool for the evaluation of possible therapeutic agents that either block protein self-association or enhance the removal of accumulated aggregates.
Footnotes
Address reprint requests to Peter Sonderegger, Department of Biochemistry, University of Zurich, Winterthurerstrasse 190, 8057 Zurich, Switzerland. E-mail: pson@bioc.unizh.ch.
Supported by the Swiss National Science Foundation and the European Union Grant APOPIS (Abnormal Proteins in the Pathogenesis of Neurodegenerative Disorders) (EU contract LSHM-CT-2003-503330) and by a grant from the Thyssen Foundation (to M.G.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Carrell RW, Lomas DA. Conformational disease. Lancet. 1997;350:134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O’Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- Carrell RW, Huntington JA. How serpins change their fold for better and for worse. Biochem Soc Symp. 2003:163–178. doi: 10.1042/bss0700163. [DOI] [PubMed] [Google Scholar]
- Lomas DA, Carrell RW. Serpinopathies and the conformational dementias. Nat Rev Genet. 2002;3:759–768. doi: 10.1038/nrg907. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Contartese J, Stoeckli ET, Kuhn TB, Sonderegger P. Neuroserpin, an axonally secreted serine protease inhibitor. EMBO J. 1996;15:2944–2953. [PMC free article] [PubMed] [Google Scholar]
- Krueger SR, Ghisu GP, Cinelli P, Gschwend TP, Osterwalder T, Wolfer DP, Sonderegger P. Expression of neuroserpin, an inhibitor of tissue plasminogen activator, in the developing and adult nervous system of the mouse. J Neurosci. 1997;17:8984–8996. doi: 10.1523/JNEUROSCI.17-23-08984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Shrimpton AE, Holohan PD, Bradshaw C, Feiglin D, Collins GH, Sonderegger P, Kinter J, Becker LM, Lacbawan F, Krasnewich D, Muenke M, Lawrence DA, Yerby MS, Shaw CM, Gooptu B, Elliott PR, Finch JT, Carrell RW, Lomas DA. Familial dementia caused by polymerization of mutant neuroserpin. Nature. 1999;401:376–379. doi: 10.1038/43894. [DOI] [PubMed] [Google Scholar]
- Davis RL, Shrimpton AE, Carrell RW, Lomas DA, Gerhard L, Baumann B, Lawrence DA, Yepes M, Kim TS, Ghetti B, Piccardo P, Takao M, Lacbawan F, Muenke M, Sifers RN, Bradshaw CB, Kent PF, Collins GH, Larocca D, Holohan PD. Association between conformational mutations in neuroserpin and onset and severity of dementia. Lancet. 2002;359:2242–2247. doi: 10.1016/S0140-6736(02)09293-0. [DOI] [PubMed] [Google Scholar]
- Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. doi: 10.1016/s0165-0270(96)00121-5. [DOI] [PubMed] [Google Scholar]
- Davis RL, Holohan PD, Shrimpton AE, Tatum AH, Daucher J, Collins GH, Todd R, Bradshaw C, Kent P, Feiglin D, Rosenbaum A, Yerby MS, Shaw CM, Lacbawan F, Lawrence DA. Familial encephalopathy with neuroserpin inclusion bodies. Am J Pathol. 1999;155:1901–1913. doi: 10.1016/S0002-9440(10)65510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman U, Wernstedt C, Gonez J, Heldin CH. Improvement of an “In-Gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem. 1995;224:451–455. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- Schrimpf SP, Langen H, Gomes AV, Wahlestedt C. A two-dimensional protein map of Caenorhabditis elegans. Electrophoresis. 2001;22:1224–1232. doi: 10.1002/1522-2683()22:6<1224::AID-ELPS1224>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Madani R, Kozlov S, Akhmedov A, Cinelli P, Kinter J, Lipp HP, Sonderegger P, Wolfer DP. Impaired explorative behavior and neophobia in genetically modified mice lacking or overexpressing the extracellular serine protease inhibitor neuroserpin. Mol Cell Neurosci. 2003;23:473–494. doi: 10.1016/s1044-7431(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Takao M, Benson MD, Murrell JR, Yazaki M, Piccardo P, Unverzagt FW, Davis RL, Holohan PD, Lawrence DA, Richardson R, Farlow MR, Ghetti B. Neuroserpin mutation S52R causes neuroserpin accumulation in neurons and is associated with progressive myoclonus epilepsy. J Neuropathol Exp Neurol. 2000;59:1070–1086. doi: 10.1093/jnen/59.12.1070. [DOI] [PubMed] [Google Scholar]
- Glatzel M, Heppner FL, Albers KM, Aguzzi A. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron. 2001;31:25–34. doi: 10.1016/s0896-6273(01)00331-2. [DOI] [PubMed] [Google Scholar]
- Inagi R, Nangaku M, Usuda N, Shimizu A, Onogi H, Izuhara Y, Nakazato K, Ueda Y, Oishi H, Takahashi S, Yamamoto M, Suzuki D, Kurokawa K, van Ypersele de Strihou C, Miyata T. Novel serpinopathy in rat kidney and pancreas induced by overexpression of megsin. J Am Soc Nephrol. 2005;16:1339–1349. doi: 10.1681/ASN.2004070600. [DOI] [PubMed] [Google Scholar]
- Takano K, Kitao Y, Inagi R, Momoi T, Matsuyama T, Miyata T, Yoneda Y, Iso H, Stern DM, Hori O, Ogawa S. A rat model of human FENIB (familial encephalopathy with neuroserpin inclusion bodies). Biochem Biophys Res Commun. 2006;346:1040–1047. doi: 10.1016/j.bbrc.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Bradshaw CB, Davis RL, Shrimpton AE, Holohan PD, Rea CB, Fieglin D, Kent P, Collins GH. Cognitive deficits associated with a recently reported familial neurodegenerative disease: familial encephalopathy with neuroserpin inclusion bodies. Arch Neurol. 2001;58:1429–1434. doi: 10.1001/archneur.58.9.1429. [DOI] [PubMed] [Google Scholar]
- Belorgey D, Sharp LK, Crowther DC, Onda M, Johansson J, Lomas DA. Neuroserpin Portland (Ser52Arg) is trapped as an inactive intermediate that rapidly forms polymers: implications for the epilepsy seen in the dementia FENIB. Eur J Biochem. 2004;271:3360–3367. doi: 10.1111/j.1432-1033.2004.04270.x. [DOI] [PubMed] [Google Scholar]
- Miranda E, Romisch K, Lomas DA. Mutants of neuroserpin that cause dementia accumulate as polymers within the endoplasmic reticulum. J Biol Chem. 2004;279:28283–28291. doi: 10.1074/jbc.M313166200. [DOI] [PubMed] [Google Scholar]
- Yazaki M, Liepnieks JJ, Murrell JR, Takao M, Guenther B, Piccardo P, Farlow MR, Ghetti B, Benson MD. Biochemical characterization of a neuroserpin variant associated with hereditary dementia. Am J Pathol. 2001;158:227–233. doi: 10.1016/S0002-9440(10)63961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrimpf SP, Bleiker AJ, Brecevic L, Kozlov SV, Berger P, Osterwalder T, Krueger SR, Schinzel A, Sonderegger P. Human neuroserpin (PI12): cDNA cloning and chromosomal localization to 3q26. Genomics. 1997;40:55–62. doi: 10.1006/geno.1996.4514. [DOI] [PubMed] [Google Scholar]
- Carlson JA, Rogers BB, Sifers RN, Finegold MJ, Clift SM, DeMayo FJ, Bullock DW, Woo SL. Accumulation of PiZ alpha 1-antitrypsin causes liver damage in transgenic mice. J Clin Invest. 1989;83:1183–1190. doi: 10.1172/JCI113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belorgey D, Crowther DC, Mahadeva R, Lomas DA. Mutant neuroserpin (S49P) that causes familial encephalopathy with neuroserpin inclusion bodies is a poor proteinase inhibitor and readily forms polymers in vitro. J Biol Chem. 2002;277:17367–17373. doi: 10.1074/jbc.M200680200. [DOI] [PubMed] [Google Scholar]
- Glatzel M, Stoeck K, Seeger H, Luhrs T, Aguzzi A. Human prion diseases: molecular and clinical aspects. Arch Neurol. 2005;62:545–552. doi: 10.1001/archneur.62.4.545. [DOI] [PubMed] [Google Scholar]