Abstract
In patients affected by Creutzfeldt-Jakob disease and in animals affected by transmissible spongiform encephalopathies, retinal functions are altered, and major spongiform changes are observed in the outer plexiform layer where photoreceptors have their synaptic terminals. In the present study, the prion protein PrPc was found to form aggregates in rod photoreceptor terminals from both rat and human retina, whereas no labeling was observed in cone photoreceptors. Discrete staining was also detected in the inner plexiform layer where the prion protein was located at human amacrine cell synapses. In mixed porcine retinal cell cultures, the PrP106-126 prion peptide triggered a 61% rod photoreceptor cell loss by apoptosis as indicated by terminal deoxynucleotidyl transferase dUTP nick-end labeling, whereas cone photoreceptors were not affected. Amacrine cells were also reduced by 47% in contrast to ganglion cells. Although this cell loss was associated with a 5.5-fold increase in microglial cells, the strict correlation between the PrPc prion protein expression and the peptide toxicity suggested that this toxicity did not rely on the release of a toxic compound by glial cells. These results provide new insights into the retinal pathophysiology of prion diseases and illustrate advantages of adult retinal cell cultures to investigate prion pathogenic mechanisms.
Transmissible spongiform encephalopathies in humans and in animals are linked to the transconformation of the prion protein PrPc into a proteinase-resistant form PrPres.1 These neurological disorders exhibit common pathological symptoms like vacuolization of the neurophils, astrocytosis, and loss of neurons.2 In patients with Creutzfeldt-Jakob disease, alteration of the retinal function was attested by the decrease in the electroretinogram b-wave amplitude,3,4,5 which reflects the activity of bipolar cells postsynaptic to photoreceptors. These early electroretinogram changes were even proposed as a diagnostic measurement for the disease.6 In histology, major spongiform changes were observed in the outer plexiform layer (OPL) where photoreceptors have their synaptic terminals, and only moderate changes were observed in the inner plexiform layer (IPL) and ganglion cell layer.5 These localizations were consistent with the reported accumulation of the pathogenic form of the prion protein, PrPres, throughout the plexiform layers of the human retina.7
The in vivo retina has been used on many occasions to study the progression of the disease in animal models because of its organized structure, its in vivo access for intraocular injection, and the possibility to correlate the histology to the measure of neuronal function by the electroretinogram. In mice inoculated with scrapie extract, the retina showed a progressive degeneration with a loss of photoreceptors and ganglion cells associated with optic nerve changes.8,9,10 These changes were correlated to a localization of the prion protein PrPc in the synaptic layers, especially in mice overexpressing the protein.11 When using prion peptides, both the PrP106-126 and PrP118-136 peptides were shown in vivo to induce cell death in all nuclear layers of the retina.12,13
When studying the molecular mechanisms of toxicity, both the PrP106-126 peptide and scrapie-infected extracts were found to have no effect on neurons from prion protein knockout mice (PrP0/0),14 whereas the PrP118-135 fragment induced neuronal cell death.13 In the presence of PrP-positive astrocytes, the PrP106-126 peptide may, however, become toxic to PrP0/0 neurons by promoting glutamate release from astrocytes.15 The toxicity of the PrP106-126 peptide was in fact related to its ability to aggregate.16
To understand further the retinal physiopathology of the transmissible spongiform encephalopathies, we examined the PrPc subcellular distribution in rat and human retina. PrPc was localized to rod spherules and amacrine cells, whereas cone pedicles did not express PrPc. In addition, we assessed the toxicity of the PrP106-126 peptide in adult mixed retinal cell cultures. A direct correlation was demonstrated between the in vitro peptide toxicity and the in vivo distribution pattern of PrPc. Mixed retinal cell cultures therefore represent an interesting model in which to study the transmissible spongiform encephalopathies physiopathological mechanisms on fully differentiated adult neurons.
Materials and Methods
Retinal Cell Culture
Adult mixed retinal cell cultures were prepared from the pig retina as previously described.17 In brief, the pig retina was isolated in a CO2-independent medium from pig eyes received from a local slaughterhouse. Retinal fragments were rinsed and incubated for 20 to 30 minutes at 37°C with activated papain (0.2%) in a Ca2+-free Ringer’s solution containing ethylenediamine tetraacetic acid (0.1 mmol/L). After stopping the enzymatic reaction in Dulbecco’s modified Eagle’s medium-10% fetal calf serum ganglion cell layer with DNase (0.2 mg/ml), retinal fragments were mechanically dissociated with a fire-polished Pasteur pipette. Retinal cells were isolated by centrifugation at 800 rpm for 5 minutes. Cells were finally seeded on laminin- and poly-d-lysine-coated coverslips in Dulbecco’s modified Eagle’s medium-10% fetal calf serum at a density of 4 × 104 cells/cm2. After 6 days in vitro, the human PrP106-126 prion peptide or its scrambled form was added to the culture medium for 4 days. Both the correct human prion protein peptide [PrP106-126 and its scrambled form were purchased from Bachem (Weil am Rhein, Germany) and applied at the same concentration (80 μmol/L)]. The 80 μmol/L concentration was selected as it has been described as the lowest toxic concentration in primary neuronal cultures.18 Peptides were dissolved in deionized water at a concentration of 24 mmol/L and stored at −20°C. Subsequently, they were diluted twice with phosphate-buffered saline (PBS) and added to the culture medium. Culture solutions were changed every 2 days, and cells were finally fixed with 4% paraformaldehyde in PBS before cell labeling.
Cell Labeling
Human postmortem retinal tissues were obtained from the human tissue bank in Strasbourg in accordance with French legislation on the use of human tissues for medical and scientific research. Human tissues were fixed in 4% paraformaldehyde in PBS (0.1 mol/L, pH 7.4) at 4°C for 15 minutes. For rat retinal tissue, adult Long-Evans rats were sacrificed by cervical dislocation. Eyes were enucleated, the anterior segments removed, and the posterior eyecups fixed in 4% paraformaldehyde in PBS for 5 minutes. Following fixation, retinal tissues were dissected from the eyecup, cryoprotected in graded sucrose solutions (10%, 20%, 30%), and embedded in OCT to produce vertical retinal sections (10 μm) on a cryostat.
Sections and cell cultures were washed in PBS, permeabilized in PBS containing 0.1% Triton X-100 for 5 minutes, then bathed in PBS containing 1% bovine serum albumin, 1% goat serum for 1 hour at 37°C, and incubated in the same solution with the primary antibody for 2 hours at room temperature. For double-labeling experiments, a combination of primary antibodies was applied simultaneously. The sections were rinsed in PBS three times and incubated with the secondary antibodies for 1 hour at 37°C in the dark. The secondary antibodies included rabbit anti-mouse IgG conjugated to Alexa TM 568 and anti-rabbit IgG conjugated to Alexa TM 488 (all diluted 1:400; Molecular Probes, Eugene OR). Microglial cells were identified by the isolectin B4 bound to fluorescein isothiocyanate (ILB4, 1:50; Sigma Chemical Co, St. Louis, MO), whereas cone photoreceptors were stained by the peanut lectin agglutinin (PNA, 1:40; Sigma). Apoptotic cells were labeled with terminal deoxynucleotidal transferase dUTP nick-end labeling (TUNEL kit; Roche Diagnostics, Basel, Switzerland). 4,6-Diamidino-2-phenylindole nuclear dye was applied in PBS for 2 minutes. Finally, cells were washed four times before observation. Control experiments were performed by either omitting the primary prion antibody or adding the primary antibody with its corresponding synthetic prion peptide.
The primary antibodies used in the present study were anti-calbindin D-28K polyclonal antibody (1:1000; Chemicon); anti-PKC-α polyclonal antibody (1:2000; Santa Cruz); anti-arrestin polyclonal antibody (1:2000; a generous gift of Dr. Y. Gery, National Eye Institute, National Institutes of Health, Bethesda, MD); anti-rhodopsin monoclonal antibody (Rho4D2, 1:1000; a generous gift of Dr. Hicks, Centre National de la Recherche Scientific Unité Mixte de Recherche 7518, Strasbourg, France); anti-syntaxin antibody (1:500, HPC1; Sigma); anti-Bassoon polyclonal antibody19 (1:1000, anti-VGLUT1 polyclonal antibody, 1:4000; a generous gift from Dr. S. El Mestikawy, INSERM U513, Créteil, France); and the anti-PrP antibodies Prion-917 (protein A-purified IgG1k, 1:600; a generous gift from Dr J. Grassi, Commissariat à l’Energie Atomique, Gif sur Yvette, France) and Prion-8G8 (IgG2ak, 1:1000; a generous gift from Dr J. Grassi). Prion-917 was raised against a synthetic peptide representative of the C-terminal human sequence (214-230) of PrP,20 whereas prion-8G8 is directed to the 90 to 108 amino acid sequence of recombinant human PrP.21 These synthetic peptides were also generously provided by Dr. J. Grassi to control for antibody specificity.
Microscopic Observation
Fluorescent labeling was observed using a Nikon Optiphot 2 microscope (Nikon, Tokyo, Japan) under epi-fluorescent illumination (Alexa TM 568: excitation filter 510 to 560 nm, dichroic mirror DM575, barrier filter BA590; Alexa TM 488: excitation filter 470 to 490 nm, dichroic mirror XF22, barrier filter 530DF30). Sections were also examined using an inverted Zeiss Axiovert 100 M microscope (Zeiss, Jena, Germany) equipped with the LSM510 laser scanning confocal module. High resolution scanning was performed with the 63×, 1.4 oil immersion objective with 1024 × 1024 or 2048 × 2048 pixel images (minimum pixel size 0.04 × 0.04 μm) in the multitrack mode. Excitation/emission wavelengths were 488 nm/505 to 530 and 543 nm/LP585 nm for Alexa TM 488 and Alexa TM 568, respectively. All images were obtained from single optical sections, 0.7 μm under blue excitation (488 nm) and 0.9 μm under green excitation (543 nm). Image processing was achieved with the LSM510 software (version 2.5) and PhotoShop 7.0 LE (Adobe Systems, San Jose, CA).
Cell Counting
In retinal cell cultures, labeled cells were counted in microscopic fields along two diagonals and normalized to the number of Rho4D2-positive rods from the same culture in the control conditions. When quantifying apoptotic rod photoreceptors, cells showing the double labeling for TUNEL and for the Rho4D2 antibody were counted. In each experiment, three coverslips were prepared and counted for each culture condition. The results are presented as the average (±SEM) of three independent cultures. Comparison between various groups of treatment was performed by Student’s t-test. A probability (P < 0.05) was taken as a landmark for significant differences.
Results
PrPc Localization in the Rat Retina
In normal rat retina, PrPc was localized with the prion-917 antibody in the OPL and IPL, where it showed a punctuated appearance (Figure 1, A and B). To control the specificity of this staining, the synthetic prion peptide used to generate the antibodies was added to the first incubation. Under such conditions, the punctuated staining in the OPL and IPL completely disappeared, leaving some nonspecific labeling of blood vessels (Figure 1C) also observed in control experiments omitting the primary antibody. To define the cellular distribution of the prion-immunopositive structures in the OPL, photoreceptor terminals were immunolabeled with an antibody directed against arrestin, a protein expressed exclusively in photoreceptors showing their terminals in the OPL and their cell bodies with inner and outer segments in the outer nuclear layer.22,23,24 In the OPL, all PrPc-immunopositive puncta were located in arrestin-immunolabeled structures, indicating that PrPc was located in photoreceptor terminals (Figure 2, A–C). To determine whether PrPc was present in cone pedicles or rod spherules, retinal sections were stained with the PNA, which labels cone pedicles in the OPL.25,26 No PrPc-immunolabeled structures were observed in PNA-positive cone pedicles (Figure 2, D–F), and there was uncertainty regarding the staining of four PNA-positive terminals of 821. This observation indicated that the PrPc was localized in rod spherules. To confirm the absence of PrPc in the dendrites of postsynaptic neurons, retinal sections were immunolabeled with an antibody directed against the α-isoform of protein kinase C (PKC-α) that specifically labels rod bipolar cells.27 Rod bipolar cell dendritic tips were often seen in close apposition with PrPc-immunolabeled structures (Figure 2, G–I). Likewise, calbindin-immunopositive horizontal cells28 were seen in close apposition to PrPc-immunolabeled structures (Figure 2, J–L). However, no co-localization was observed, confirming the presynaptic localization of the PrPc in rat rod photoreceptors.
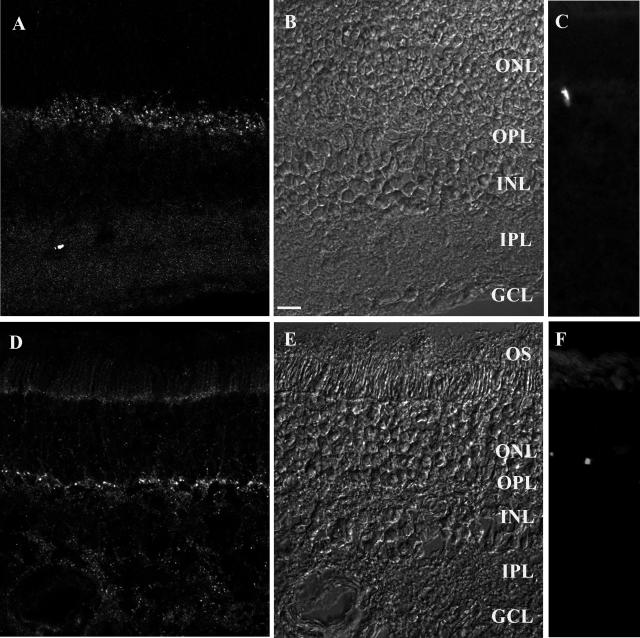
Figure 1.
Prion PrPc protein distribution in the rat (A–C) and human (D–F) retina. Retinal sections immunolabeled for the PrPc protein (A, D) and visualized under Normarski optics (B, E). In both the rat (A) and human (D) retina, the PrPc-immunopositive structures were present in the outer and inner plexiform layer (OPL, IPL), showing a punctuated appearance. In control experiments with synthetic prion peptides, the PrPc-immunopositive structures were no longer visible, leaving unspecific labeling of blood vessels in the rat (C) and human (F) retina. The scale bars in B represent 10 μm. OS, outer segment; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
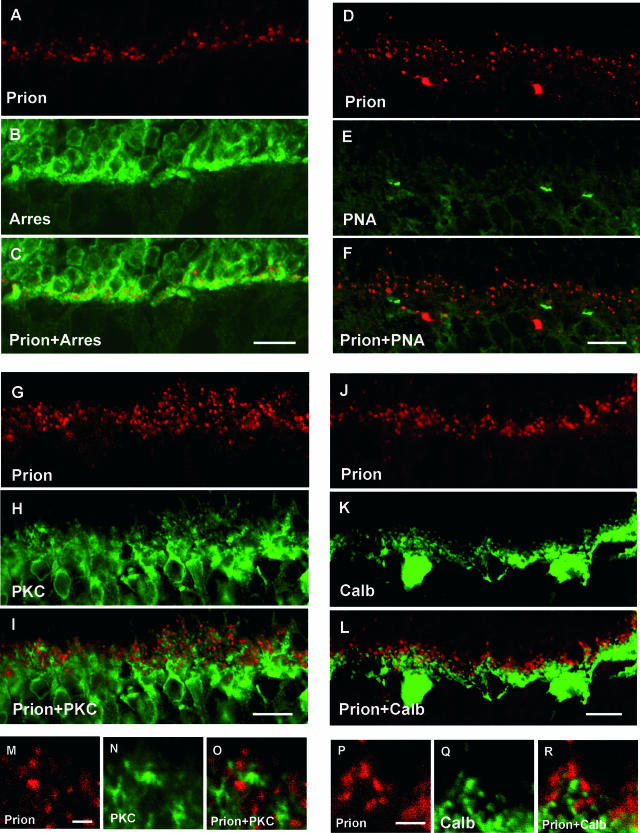
Figure 2.
Subcellular localization of the prion PrPc protein in the rat OPL. Confocal microscopic observations of rat retinal sections immunolabeled for the prion protein (red in A, C, D, F, G, I, J, L, M, O, P, R), arrestin (Arres, green in B, C), peanut agglutinin lectin (PNA, green in E, F), PKC-α, (PKC, green in H, I, N, O), calbindin (Calb, green in K, L, Q, R). The prion protein was co-localized with the arrestin labeling of the photoreceptor terminals (A–C). No prion-immunopositive structures were associated with the PNA staining of cone photoreceptor terminals (D–F). PrPc-immunopositive structures were in apposition to PKC-α-positive rod bipolar cell dendrites (G–I, M–O) and calbindin-positive horizontal cell tips (J–L; P–R). Scale bars represent 10 μm in A–L and 2 μm in M–R.
PrPc Localization in the Human Retina
Likewise, PrPc was localized with the prion-8G8 antibody in the human OPL and IPL as punctuated structures (Figure 1, D–E). Again, the specificity of this staining was controlled with the synthetic prion peptide used to generate the antibodies. With this peptide, the punctuated staining in the OPL and IPL completely disappeared, leaving some unspecific labeling of blood vessels (Figure 1F). These PrPc-immunopositive structures were included within the arrestin-positive photoreceptor terminals (Figure 3, A–C). To confirm further this photoreceptor expression, retinal sections were labeled with an antibody recognizing the vesicular glutamate transporter VGLUT1, which contributes to the vertical excitatory transmission at photoreceptor and bipolar cell terminals. In the OPL, all PrPc-immunopositive structures were included within VGLUT1-immunopositive photoreceptor terminals (Figure 3, D–F). When cone photoreceptor pedicles were distinguished by the PNA labeling, PrPc immunolabeling was not found in these cone photoreceptor terminals (Figure 3, J–L). To confirm further the absence of PrPc in cone pedicles, retinal sections were immunolabeled with an antibody directed against calbindin that labels the complete cone photoreceptor in the human retina.28 No PrPc immunolabeling was observed in calbindin-positive cone photoreceptor terminals (Figure 4, A–C). In the OPL, PrPc-immunopositive structures were in close apposition with rod bipolar cell dendritic tips as revealed by PKC-α immunolabeling (Figure 3, G–I). As in the rat retina, these observations indicate that PrPc is expressed in human rod spherules but not in cone photoreceptor pedicles.
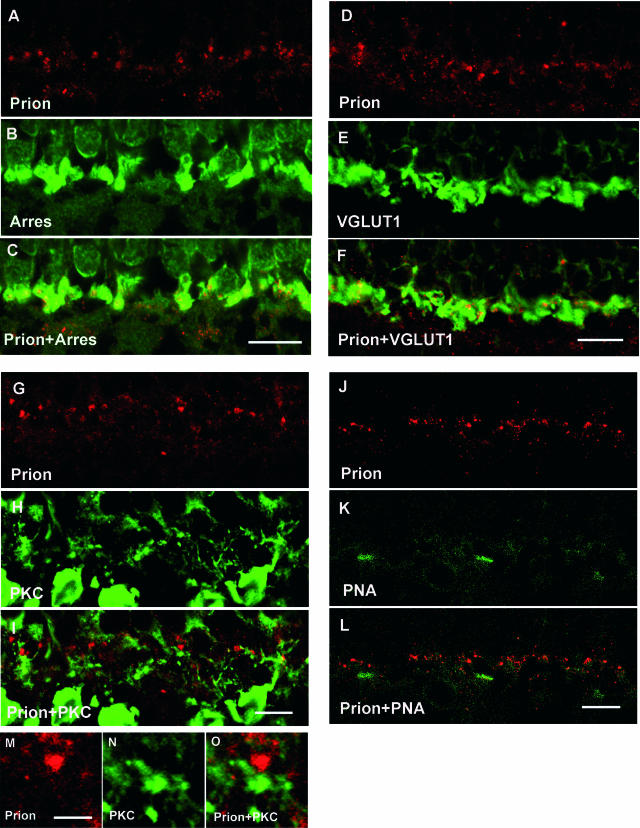
Figure 3.
Subcellular localization of the prion PrPc protein in the human outer plexiform layer. Confocal microscopic observations of human retinal sections immunolabeled for the prion protein (red in A, C, D, F, G, I, J, L, M, O), arrestin (Arres, green in B, C), vesicular glutamate transporter 1 (VGLUT1, green in E, F), PKC-α (PKC, green in H, I, N, O), and peanut agglutinin lectin (PNA, green in K, L). The PrPc-immunopositive puncta (A, D) were localized within the arrestin-positive (B, C) and VGLUT1-positive photoreceptor terminals (E–F) as indicated on the merged images (C, F). These PrPc-positive puncta (G, M) were in close apposition to PKC-α-positive rod bipolar cell dendrites (H, I, N, O). By contrast, the PrPc-positive structures (J) were not associated with the PNA staining of cone photoreceptor terminals (K, L). Scale bars represent 10 μm in A–L and 2 μm in M–O.
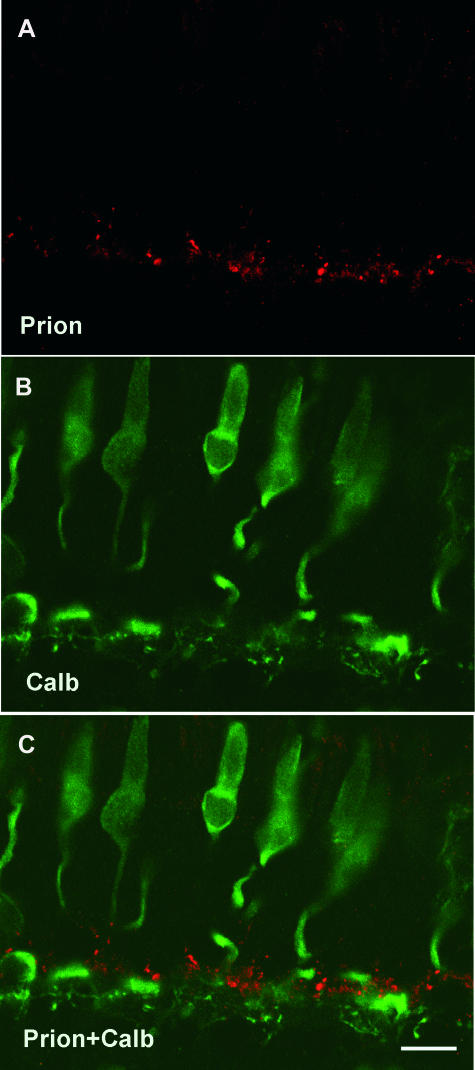
Figure 4.
Absence of the PrPc prion protein in human cone photoreceptors. Confocal microscopic observations of human retinal sections immunolabeled for the PrPc protein (red in A, C) and calbindin (Calb, green in B, C). The PrPc-positive puncta were not localized with the calbindin labeling of cone photoreceptors (C). The scale bar in C represents 10 μm.
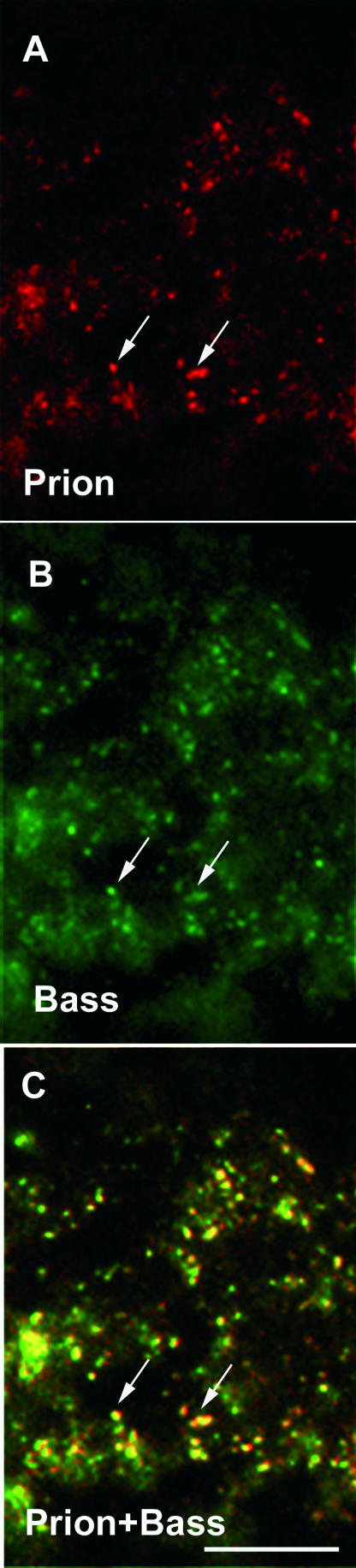
To define the subcellular localization of PrPc in the IPL, retinal sections were labeled with a Bassoon antibody that recognizes the conventional synapses of inhibitory amacrine cells.29 PrPc-immunopositive structures were co-localized with Bassoon-immunopositive structure (Figure 5, A–C), thereby indicating that the PrPc protein is located at the amacrine cell synapse.
Figure 5.
Subcellular localization of the prion PrPc protein in the human inner plexiform layer. Confocal microscopic observations of human retinal sections immunolabeled for the PrPc protein (red in A, C) and Bassoon (Bass, green in B, C). The PrPc-positive puncta were co-localized with the Bassoon labeling of inhibitory amacrine cell synaptic terminals in the IPL (arrow in A–C). The scale bar represents 5 μm in A–C.
Neurotoxicity of Prion Protein Fragment 106-126
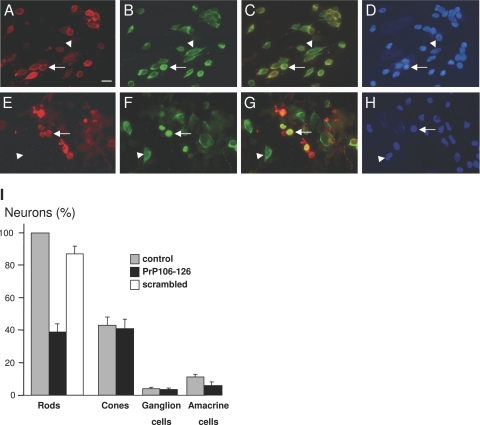
In the in vivo retina, the peptide PrP106-126 was previously reported to induce neuronal death in all retinal layers.12 To examine further the retinal toxicity of the PrP106-126 peptide, mixed retinal cell cultures from the pig retina were exposed to this peptide for 4 days. The choice of the pig retina for these culture experiments was taken for ethical reasons because pig eyes are available from the slaughterhouse. Furthermore, pigs have many more cone photoreceptors, enabling us to investigate the toxicity of the PrP106-126 peptide on PrPc-expressing rods compared with cones that do not express PrPc. Figure 6 illustrates the observed neuronal cell loss after 4-day exposure to the PrP106-126. In these experiments, cell populations were normalized with respect to the number of rod photoreceptors in the control condition, photoreceptors were predominant with the cones representing a third of photoreceptors (cone/rod ratio: 43.21 ± 5.11%, SEM, n = 3), whereas amacrine and ganglion cells constituted minor cell populations (amacrine cell/rod ratio: 11.36 ± 1.40%; ganglion cell/rod ratio: 3.86 ± 0.74%, SEM, n = 3). When treated with the P106-126 peptide, rods were decreased by 61.27 ± 4.98% (SEM, n = 3) in the PrP106-126-treated group. The specificity of this rod neurotoxicity was investigated with a scrambled PrP106-126 peptide. Unlike the P106-126 peptide, this scrambled peptide did not induce a similar cell loss at the same concentration (Figure 6, rod cell number with respect to control: 87.83 ± 4.69%, SEM, n = 3). Interestingly, the P106-126 peptide did not induce cone cell death as no significance difference in the relative cone cell numbers were observed between the control group (43.21 ± 5.11%, SEM, n = 3) and PrP106-126-treated group (40.96 ± 6.15%, SEM, n = 3). Likewise, no significant differences were detected for ganglion cells between the control group (3.86 ± 0.74%, SEM, n = 3) and the PrP106-126-treated group (3.45 ± 0.72%, SEM, n = 3). By contrast, syntaxin-immunopositive amacrine cells were significantly decreased by 47.01 ± 4.36% (SEM, n = 3), following the peptide treatment. These results indicated that the PrP106-126 peptide induced cell death in cultured rod photoreceptors and amacrine cells, although it did not affect cone photoreceptors and ganglion cells.
Figure 6.
The prion peptide PrP106-126 induced rod cell death in mixed retinal culture. Control (A–D) and PrP106-126-treated retinal cell cultures (E–H) that were immunolabeled for rhodopsin (red in A, C, E, G) and arrestin (green in B, C, F, G) and stained with the nuclear dye 4,6-diamidino-2-phenylindole (D, H). Rod photoreceptors exhibited rhodopsin labeling of plasma membranes and arrestin staining of the cytoplasm (arrow in A–D), whereas cone photoreceptors were specifically identified by the arrestin antibody (arrowheads in A–H). Note in the PrP106-126 treated cultures that some structures showed an abnormally intense rhodopsin immunolabeling (arrow in E–H) but lacked the 4,6-diamidino-2-phenylindole nuclear staining. I: Quantification of retinal cells in control retinal cell cultures (control), cultures treated with the PrP106-126 peptide (PrP106-126), and culture treated with a scrambled peptide. The differences in rod and amacrine cell survival were statistically significant (P < 0.05). All measures were normalized to the number of rods in the control conditions and provided as SEM with n = 3 for both conditions. The scale bar in A represents 10 μm.
To provide further evidence of rod cell death, treated cultures were labeled for the apoptotic marker TUNEL. Double-labeling experiment with TUNEL and the rod-specific antibody Rho4D2 showed that many rods were TUNEL-positive cells. Quantification of these TUNEL-positive rod cells showed an increase in density from 23.44 ± 3.90% to 62.17 ± 8.97% of the rod cell population (SEM, n = 3). TUNEL-positive photoreceptors often showed an increased intensity of the rhodopsin immunolabeling that is normally homogeneously distributed at the cell membrane. Fragmentation or absence of the cell nuclei was also occasionally observed in PrP106-126-treated rod photoreceptors (Figure 6). These results confirmed that the PrP106-126 peptide can induce rod photoreceptor apoptosis in mixed retinal culture.
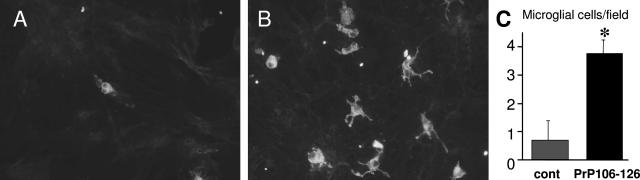
Activation of microglia was reported following culture incubation with the PrP106-126 peptide.14 To determine whether this microglial reaction occurred in retinal cell culture, microglial cells were labeled with the isolectin B4 and counted. The ILB4 lectin staining showed a change in the microglial morphology following the PrP106-126 treatment (Figure 7). Control microglia were oval-shaped, showing no or a very few processes, whereas PrP106-126-treated microglia exhibited several cellular processes resulting in a star-like cellular morphology. Furthermore, cell quantification indicated that microglia had increased 5.5-fold from 0.68 ± 0.10 cells/field to 3.74 ± 0.50 cells/field (SEM, n = 3) following the PrP106-126 peptide treatment. These results were consistent with an activation and proliferation of retinal microglial cells in the presence of the PrP106-126 peptide.
Figure 7.
Microglial cell increase in mixed retinal cell cultures incubated in the presence of the PrP106-126 prion peptide. A and B: Microglial cell staining by the isolectin B4 lectin (ILB4) in control (A) and PrP106-126-treated retinal cell cultures (B). An increase in the number of microglia and a change of their morphology were observed after 4 days of treatment with PrP106-126. Control microglia were oval-shaped, showing no or a very few processes, whereas PrP106-126-treated microglial cells exhibited several cellular processes conferring a star-like morphology on the cell. C: Quantification of ILB4-labeled microglia in control retinal culture or culture treated with the PrP106-126 peptide. The difference in the number of microglial cells was statistically significant when applying the PrP106-126 prion peptide (*P < 0.05, SEM, n = 3). The scale bar in A represents 10 μm.
Discussion
PrPc Localization in the Retina
In the retina, PrPc expression was located in all nuclear layers by in situ hybridization.7 The protein was localized in the plexiform layers from transgenic mice overexpressing the protein PrPc.30,31 Its co-localization with synaptophysin suggested that it had a presynaptic localization in the retina as in other neuronal structures, although it did not seem to be restricted to the plasma membrane as it had been suggested from synaptosomal fractionation studies.11 Our histological examinations confirmed that PrPc was indeed presynaptic in the outer plexiform layer, but it indicated further that in both rat and human retina it was selectively expressed in rod photoreceptor terminal and not in cone pedicles. However, the immunolabeling did not show a diffuse staining of the terminals as reported in the transgenic animals overexpressing the protein, but it appeared instead as dense puncta. It was consistent with the intense labeling of the outer plexiform layer obtained for the proteinase K-resistant protein (PrPres) in patients affected by the Creutzfeldt-Jakob disease7 or in infected transgenic mice.32 It could further explain the disappearance or spongiform aspect of the outer plexiform layer in patients with Creutzfeldt-Jakob disease.5 PrPc was also located in amacrine cell terminals, consistent with previous observations of the native and protease-resistant protein in the IPL.7,32,33 This location could also explain the histological damage occasionally observed in the IPL of patients affected by Creutzfeldt-Jakob disease.5
PrP106-126 Peptide-Induced Neuronal Toxicity
The PrP106-126 peptide served as a suitable model of the PrPres neurotoxicity because, as for infectious scrapie strains,34 it is toxic to PrPc protein-expressing neurons but not to neurons from PrP0/0 mice.14,35 In the retina, PrP106-126 intravitreal injections decreased both the electroretinogram a- and b-wave amplitudes, thereby indicating dysfunction both in photoreceptors and in their postsynaptic neurons.12 Retinal cells in all layers were undergoing apoptosis as demonstrated by TUNEL labeling.12 Our in vitro observations showed a selective cell death to rod photoreceptors and amacrine cells. As in cerebellar neurons,14 this specific cell toxicity seemed to relate to the PrPc expression because, in contrast to cones, rods exhibited an intense labeling of their terminals and were sensitive to the peptide toxicity. The absence of cone and ganglion cell death may seem to contradict the reported peptide toxicity in all retinal layers in vivo.12 However, rods represent the most abundant cells in the outer nuclear layer, whereas amacrine cells constitute a major cell population in both the inner nuclear layer and ganglion cell layer. Therefore, the toxicity on these two cell types could explain the reported histological features of the retina following intraocular injections of the peptide. Other secondary mechanisms, such as the release of excitotoxic concentrations of glutamate,15 could also explain the greater in vivo damages.
Implications of Glial Cells in the PrP106-126 Peptide-Induced Neuronal Toxicity
As for scrapie-infected homogenates,36 the PrP106-126 peptide toxicity was reported to rely on the presence of reactive PrPc-positive microglial cells.14,31 Microglial cells activated by PrP106-126 were, however, unable to induce cell death in cerebellar neurons from PrP0/0 mice.14 Scrapie-activated or PrP106-126 peptide-activated microglial cells were shown to release toxic molecules for neurons including nitrites, anion superoxide, and other mediators of the inflammatory response.36,37 In our mixed retinal cell cultures, microglial cells seemed to proliferate during the PrP106-126 peptide incubation, as previously reported with microglial cells from other tissues.14 The PrPres-induced increase in retinal microglial cells reported in vivo36 could have arisen from cell recruitment at the optic nerve or alternatively from proliferation of resident cells. However, the selective induction of cell death in PrPc-positive rods but not in PrPc-negative cones suggested that rod toxicity was not mediated by an unspecific toxic molecule. The PrP cell specificity could be related to the selective expression of a receptor for the inflammatory response. The PrP106-126 neuronal cell toxicity was also attributed to astroglial cells releasing glutamate in the neuronal co-culture.15 Although both glial Müller cells and astrocytes were present in our cultures, a toxic glutamate release is unlikely to be the mechanism of toxicity because rod photoreceptors do not express excitatory glutamate receptors and ganglion cells would have been affected.
Retinal Cell Pathology in Prion Diseases
Few histopathological examinations of ocular tissues have been performed in humans.4,5,38,39 Tsutsui et al39 reported a case with posterior central retinal degeneration with loss of ganglion cells and nerve fibers and vacuolization of the outer plexiform layer. Lesser et al38 reported severe optic neuropathy associated with visual loss and ganglion cell degeneration. In other cases of Creutzfeldt-Jakob disease, the ophthalmologic examination was often normal.5 The visual symptoms were related to supranuclear or internuclear oculomotor paresis, visual agnosia, palinopsia, hemianopsia. In histology, inner and outer photoreceptor segments were well preserved, but significant spongiform changes were identified in the OPL. Our PrPc localization in rod terminals is consistent with these spongiform changes in the OPL and the early decrease in the electroretinogram b-wave amplitude in several cases of Creutzfeldt-Jakob disease.3,4,5,6 The b-wave is indeed generated by bipolar cells postsynaptic to photoreceptors, and its decrease is indicative of a dysfunction in photoreceptor synaptic transmission or in bipolar cell impairment. The spongiform changes in the OPL were more prominent in the peripheral than in the central part of the retina.5 The specific localization of PrPc aggregates in rod terminals and their absence in cone photoreceptors may explain this peripheral to central gradient of OPL vacuolization, which follows the rod/cone ratio.
Conclusions
This study on the retinal distribution of PrPc and on the PrP106-126 retinal cell toxicity is consistent with the pathological observations in patients with Creutzfeldt-Jakob disease. Retinal cell cultures may offer some advantages in the future to understand the physiopathology of prion diseases, because these cultures can be prepared from adult tissues and even from human adult postmortem retinal tissues.17
Acknowledgments
We thank Dr. J. Grassi for the 8G8 antibody, the prion-917 antibody, and the corresponding synthetic prion peptides; Dr. D. Hicks for the rho4D2 antibody; Dr. S. El Mestikawy for the VGLUT1 antibody; Dr. Y. Gery for the arrestin antibody; and Dr. Cronin for providing comments on the manuscript.
Footnotes
Address reprint requests to Serge Picaud, INSERM U592, Bâtiment Kourilsky, 184 rue du Faubourg Saint-Antoine, F-75571 Paris Cedex 12, France. E-mail: picaud@st-antoine.inserm.fr.
Supported by the GIS prion, INSERM ATC-prion, the University Louis Pasteur (Strasbourg), the University Pierre and Marie Curie (Paris VI), the Fédération des Aveugles de France, and the European Economic community (EVI-GENORET-512036). J.G. received fellowships from the Fédération des Aveugles de France, l’Association Information et Recherche sur la Rétinite Pigmentaire, and France-Regard.
References
- Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser H. Diversity in the neuropathology of scrapie-like diseases in animals. Br Med Bull. 1993;49:792–809. doi: 10.1093/oxfordjournals.bmb.a072647. [DOI] [PubMed] [Google Scholar]
- Renault F, Richard P. Early electroretinogram alterations in Creutzfeldt-Jakob disease after growth hormone treatment. Lancet. 1991;338:191. doi: 10.1016/0140-6736(91)90185-r. [DOI] [PubMed] [Google Scholar]
- Katz BJ, Warner JE, Digre KB, Creel DJ. Selective loss of the electroretinogram B-wave in a patient with Creutzfeldt-Jakob disease. J Neuroophthalmol. 2000;20:116–118. doi: 10.1097/00041327-200020020-00011. [DOI] [PubMed] [Google Scholar]
- de Seze J, Hache JC, Vermersch P, Arndt CF, Maurage CA, Pasquier F, Laplanche JL, Ruchoux MM, Leys D, Destee A, Petit H. Creutzfeldt-Jakob disease: neurophysiologic visual impairments. Neurology. 1998;51:962–967. doi: 10.1212/wnl.51.4.962. [DOI] [PubMed] [Google Scholar]
- Richard P, Renault F, Ostre C, Auzoux-Cheve M. Neurophysiological follow-up in two children with Creutzfeldt-Jakob disease after human growth hormone treatment. Electroencephalogr Clin Neurophysiol. 1994;91:100–107. doi: 10.1016/0013-4694(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Head MW, Northcott V, Rennison K, Ritchie D, McCardle L, Bunn TJ, McLennan NF, Ironside JW, Tullo AB, Bonshek RE. Prion protein accumulation in eyes of patients with sporadic and variant Creutzfeldt-Jakob disease. Invest Ophthalmol Vis Sci. 2003;44:342–346. doi: 10.1167/iovs.01-1273. [DOI] [PubMed] [Google Scholar]
- Buyukmihci N, Rorvik M, Marsh RF. Replication of the scrapie agent in ocular neural tissues. Proc Natl Acad Sci USA. 1980;77:1169–1171. doi: 10.1073/pnas.77.2.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, Fraser H, Foster JD, Scott JR. The correlation of electroretinographic and histopathological findings in the eyes of mice infected with the 79A strain of scrapie. Neuropathol Appl Neurobiol. 1989;15:75–89. doi: 10.1111/j.1365-2990.1989.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Russelakis-Carneiro M, Betmouni S, Perry VH. Inflammatory response and retinal ganglion cell degeneration following intraocular injection of ME7. Neuropathol Appl Neurobiol. 1999;25:196–206. doi: 10.1046/j.1365-2990.1999.00184.x. [DOI] [PubMed] [Google Scholar]
- Herms J, Tings T, Gall S, Madlung A, Giese A, Siebert H, Schurmann P, Windl O, Brose N, Kretzschmar H. Evidence of presynaptic location and function of the prion protein. J Neurosci. 1999;19:8866–8875. doi: 10.1523/JNEUROSCI.19-20-08866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettaiche M, Pichot R, Vincent JP, Chabry J. In vivo cytotoxicity of the prion protein fragment 106-126. J Biol Chem. 2000;275:36487–36490. doi: 10.1074/jbc.C000579200. [DOI] [PubMed] [Google Scholar]
- Chabry J, Ratsimanohatra C, Sponne I, Elena PP, Vincent JP, Pillot T. In vivo and in vitro neurotoxicity of the human prion protein (PrP) fragment P118-135 independently of PrP expression. J Neurosci. 2003;23:462–469. doi: 10.1523/JNEUROSCI.23-02-00462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Schmidt B, Kretzschmar HA. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature. 1996;380:345–347. doi: 10.1038/380345a0. [DOI] [PubMed] [Google Scholar]
- Brown DR. Prion protein peptide neurotoxicity can be mediated by astrocytes. J Neurochem. 1999;73:1105–1113. doi: 10.1046/j.1471-4159.1999.0731105.x. [DOI] [PubMed] [Google Scholar]
- Rymer DL, Good TA. The role of prion peptide structure and aggregation in toxicity and membrane binding. J Neurochem. 2000;75:2536–2545. doi: 10.1046/j.1471-4159.2000.0752536.x. [DOI] [PubMed] [Google Scholar]
- Picaud S, Hicks D, Forster V, Sahel J, Dreyfus H. Adult human retinal neurons in culture: physiology of horizontal cells. Invest Ophthalmol Vis Sci. 1998;39:2637–2648. [PubMed] [Google Scholar]
- White AR, Guirguis R, Brazier MW, Jobling MF, Hill AF, Beyreuther K, Barrow CJ, Masters CL, Collins SJ, Cappai R. Sublethal concentrations of prion peptide PrP106-126 or the amyloid beta peptide of Alzheimer’s disease activates expression of proapoptotic markers in primary cortical neurons. Neurobiol Dis. 2001;8:299–316. doi: 10.1006/nbdi.2001.0386. [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Sanmarti-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kampf U, Franzer JT, Stumm M, Garner CC, Gundelfinger ED. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demart S, Fournier JG, Creminon C, Frobert Y, Lamoury F, Marce D, Lasmezas C, Dormont D, Grassi J, Deslys JP. New insight into abnormal prion protein using monoclonal antibodies. Biochem Biophys Res Commun. 1999;265:652–657. doi: 10.1006/bbrc.1999.1730. [DOI] [PubMed] [Google Scholar]
- Krasemann S, Groschup MH, Harmeyer S, Hunsmann G, Bodemer W. Generation of monoclonal antibodies against human prion proteins in PrP0/0 mice. Mol Med. 1996;2:725–734. [PMC free article] [PubMed] [Google Scholar]
- Faure JP, Mirshahi M, Dorey C, Thillaye B, de Kozak Y, Boucheix C. Production and specificity of monoclonal antibodies to retinal S antigen. Curr Eye Res. 1984;3:867–872. doi: 10.3109/02713688409000800. [DOI] [PubMed] [Google Scholar]
- von Schantz M, Szel A, van Veen T, Farber DB. Expression of soluble phototransduction-associated proteins in ground squirrel retina. Invest Ophthalmol Vis Sci. 1994;35:3922–3930. [PubMed] [Google Scholar]
- Mirshahi M, Boucheix C, Collenot G, Thillaye B, Faure JP. Retinal S-antigen epitopes in vertebrate and invertebrate photoreceptors. Invest Ophthalmol Vis Sci. 1985;26:1016–1021. [PubMed] [Google Scholar]
- Sameshima M, Uehara F, Ohba N. Specialization of the interphotoreceptor matrices around cone and rod photoreceptor cells in the monkey retina, as revealed by lectin cytochemistry. Exp Eye Res. 1987;45:845–863. doi: 10.1016/s0014-4835(87)80101-x. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Johnson LV. Biochemical characterization of the major peanut-agglutinin-binding glycoproteins in vertebrate retinae. J Comp Neurol. 1986;249:499–510. doi: 10.1002/cne.902490406. 482–483. [DOI] [PubMed] [Google Scholar]
- Wässle H, Yamashita M, Greferath U, Grunert U, Muller F. The rod bipolar cell of the mammalian retina. Vis Neurosci. 1991;7:99–112. doi: 10.1017/s095252380001097x. [DOI] [PubMed] [Google Scholar]
- Hamano K, Kiyama H, Emson PC, Manabe R, Nakauchi M, Tohyama M. Localization of two calcium binding proteins, calbindin (28 kD) and parvalbumin (12 kD), in the vertebrate retina. J Comp Neurol. 1990;302:417–424. doi: 10.1002/cne.903020217. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wassle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Strome R, Carlson GA, Westaway D. Syrian hamster prion protein (PrP(C)) is expressed in photoreceptor cells of the adult retina. Neurosci Lett. 1997;234:11–14. doi: 10.1016/s0304-3940(97)00669-1. [DOI] [PubMed] [Google Scholar]
- Herms JW, Madlung A, Brown DR, Kretzschmar HA. Increase of intracellular free Ca2+ in microglia activated by prion protein fragment. Glia. 1997;21:253–257. doi: 10.1002/(sici)1098-1136(199710)21:2<253::aid-glia8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kercher L, Favara C, Chan CC, Race R, Chesebro B. Differences in scrapie-induced pathology of the retina and brain in transgenic mice that express hamster prion protein in neurons, astrocytes, or multiple cell types. Am J Pathol. 2004;165:2055–2067. doi: 10.1016/S0002-9440(10)63256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head MW, Peden AH, Yull HM, Ritchie DL, Bonshek RE, Tullo AB, Ironside JW. Abnormal prion protein in the retina of the most commonly occurring subtype of sporadic Creutzfeldt-Jakob disease. Br J Ophthalmol. 2005;89:1131–1133. doi: 10.1136/bjo.2004.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Kretzschmar HA, Giese A, Brown DR, Herms J, Keller B, Schmidt B, Groschup M. Cell death in prion disease. J Neural Transm Suppl. 1997;50:191–210. doi: 10.1007/978-3-7091-6842-4_19. [DOI] [PubMed] [Google Scholar]
- Marella M, Chabry J. Neurons and astrocytes respond to prion infection by inducing microglia recruitment. J Neurosci. 2004;24:620–627. doi: 10.1523/JNEUROSCI.4303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser RL, Albert DM, Bobowick AR, O’Brien FH. Creutzfeldt-Jakob disease and optic atrophy. Am J Ophthalmol. 1979;87:317–321. doi: 10.1016/0002-9394(79)90070-9. [DOI] [PubMed] [Google Scholar]
- Tsutsui J, Kawashima S, Kajikawa I, Shirabe T, Terao A. Electrophysiological and pathological studies on Creutzfeldt-Jakob disease with retinal involvement. Doc Ophthalmol. 1986;63:13–21. doi: 10.1007/BF00153007. [DOI] [PubMed] [Google Scholar]