Abstract
Tumor cell-induced platelet aggregation has been reported to facilitate hematogenous metastasis. Aggrus/podoplanin is a platelet aggregation-inducing factor that is up-regulated in a number of human cancers and has been implicated in tumor progression. We studied herein the role of Aggrus in tumor growth, metastasis, and survival in vivo. Aggrus expression in Chinese hamster ovary cells promoted pulmonary metastasis in both an experimental and a spontaneous mouse model. No differences in the size of metastatic foci or in primary tumor growth were found in either set of mice. Aggrus-expressing cells, which were covered with platelets, arrested in the lung microvasculature 30 minutes after injection. In addition, lung metastasis resulting from Aggrus expression decreased the survival of the mice. By generating several Aggrus point mutants, we revealed that point mutation at the platelet aggregation-stimulating domain of Aggrus (Thr34 and Thr52) obliterated both platelet aggregation and metastasis. Furthermore, administration of aspirin to mice reduced the number of metastatic foci. These results indicate that Aggrus contributes to the establishment of metastasis by promoting platelet aggregation without affecting subsequent growth. Thus, Aggrus could serve as an ideal therapeutic target for drug development to block metastasis.
Metastasis is the major cause of death from cancer, yet the optimal strategy against it remains uncertain. The pathogenesis of metastasis is dynamic and consists of the following steps: 1) cellular transformation and tumor growth, 2) angiogenesis, 3) detachment and local invasion of the host stroma, 4) entry into the bloodstream, 5) transport along circulation, 6) arrest in the capillary, 7) extravasation, and 8) proliferation within the foreign tissue.1 However, even if tumor cells successfully proceed through steps 1 to 3 and get into the circulation, almost all circulating tumor cells are rapidly destroyed by the shear forces or are attacked by the immune system, and less than 0.01% of these cells survive to produce metastasis.2 Thus, a key to success in metastasis relies on tumor survival in the bloodstream. One of the strategies by which tumor cells achieve this is platelet aggregation, ie, tumor cell-induced platelet aggregation (TCIPA).
Platelet aggregation is believed to protect tumor cells from immunological assault in the circulation. Indeed, it has been shown that platelets protect tumors from tumor necrosis factor α-mediated cytotoxicity.3 Another survival advantage is the tendency for the large tumor-platelet aggregate to embolize the microvasculature at a new extravasation site.4 Platelets also facilitate the adhesion of tumor cells to the vascular endothelium5 (step 6) and release a number of growth factors that promote tumor cell growth. Mice without platelets, because of genetic elimination of Nf-E2, showed marked protection against metastasis.6 Recently, it has been reported that platelets contribute to tumor-induced angiogenesis by releasing angiogenic growth factors, such as vascular endothelial growth factor.7,8,9 There are several mechanisms involved in TCIPA, and these can vary among different tumor cells. For example, tumor cells can activate platelets by tumor cell-induced thrombin generation through a coagulation pathway,10,11 releasing adenosine 5′-diphosphate (ADP),12 thromboxane A2,13 matrix metalloproteinase 2,14 and the membrane protein Aggrus.15
Aggrus/podoplanin is a type I transmembrane sialomucin-like glycoprotein that consists of an extracellular domain with abundant serine and threonine residues as potential O-glycosylation sites, a single transmembrane portion, and a short cytoplasmic tail with putative sites for protein kinase C and cAMP phosphorylation.16 Because Aggrus/podoplanin is expressed on the lymphatic endothelium but not on blood vessel endothelium, it is also widely used in histopathology as a specific marker for lymphatic endothelium and lymphangiogenesis.17 Aggrus expression has been shown to be up-regulated in a number of different cancers, including squamous cell carcinomas (oral cavity,16 lung,18 skin,19 and head and neck20), granulosa cell tumors,19 mesotheliomas,21 testicular seminomas,22 central nervous system tumors,23,24,25 and lobular breast cancers,26 suggesting that increased expression of Aggrus is associated with tumor malignancy and poor clinical outcome.20 We previously showed expression of Aggrus-induced platelet aggregation with no requirement for plasma components.15 We also identified the segment of EDxxVTPG in the extracellular domain as the platelet aggregation-stimulating (PLAG) domain, which is critical for the platelet-aggregating activity of Aggrus.15 Aggrus contains three tandem repeats of the PLAG domain.27 In addition, these PLAG domains were highly conserved among Aggrus homologues from human, mouse, rat, dog, and hamster.27 However, it is yet to be elucidated whether its platelet-aggregating activity is directly involved in the in vivo metastasis-forming activity.
In this study, we investigated the role of human Aggrus in tumor growth, metastasis, and survival. We established Chinese hamster ovary (CHO) cells, which had been stably transfected with wild-type (WT) Aggrus or its PLAG domain mutants. We discovered that Aggrus expression promoted pulmonary metastasis in both experimental and spontaneous metastasis models and decreased survival of the mice. Platelet aggregation-inducing activity of Aggrus is directly associated with metastasis formation because introducing a point mutation into the PLAG domains or administration of aspirin to mice decreased the formation of pulmonary metastasis.
Materials and Methods
Cell Culture Conditions
CHO cells were cultured in RPMI 1640 medium (Nissui, Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St. Louis, MO), 2 mmol/L l-glutamine (Life Technologies, Inc., Grand Island, NY), and 100 μg/ml kanamycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Animals
Female BALB/c mice (5 weeks old) and BALB/c-nu/nu mice (5 weeks old) were purchased from Charles River Japan, Inc. (Kanagawa, Japan). Animals were housed under pathogen-free conditions. The Animal Care and Use Committee of the Institute of Molecular and Cellular Biosciences approved the animal experiments described herein.
Establishment of CHO Cells Stably Expressing WT or Mutant Aggrus Proteins
The pcDNA3 vector containing WT human Aggrus cDNA was established in our laboratory, as described previously.15 Substitution of the appropriate threonine codons to alanine codons in human Aggrus cDNA was accomplished using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). CHO cells were then transfected with the plasmids using LipofectAMINE 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Stable transfectants were selected by cultivating the cells in the medium containing 1 mg/ml Geneticin (G418; Sigma). We used two independent clones of each Aggrus-expressing clones. The expression level of human Aggrus was confirmed by Western blot analysis.
Western Blot Analysis
Cultured cell pellets or mice tumors were solubilized with lysis buffer (25 mmol/L Tris, pH 7.4, 50 mmol/L NaCl, 0.5% sodium deoxycholate, 2% Nonidet P-40, 0.2% sodium dodecyl sulfate, 1 mmol/L phenylmethyl sulfonyl fluoride, and 50 mg/ml aprotinin), electrophoresed, and blotted onto a nitrocellulose membrane. The membranes were incubated with an anti-human Aggrus YM-1 monoclonal antibody (hybridoma culture supernatant)28 or an anti-β-actin antibody (Sigma). Membranes were subsequently washed and incubated with horseradish peroxidase-conjugated secondary antibody. After washing, the membranes were developed with an enhanced chemiluminescence system, according to the manufacturer’s instructions (GE Health Care UK Ltd., Buckinghamshire, UK).
Flow Cytometry
The cell surface expression of WT-Aggrus and Aggrus point mutants was confirmed by flow cytometric analysis. Cells were harvested by brief exposure to trypsin. After washing with phosphate-buffered saline, cells were treated with YM-1 monoclonal antibody for 1 hour at 4°C, and then cells were incubated with Oregon Green 488-conjugated antibody (Invitrogen Molecular Probes, Eugene, OR) for 30 minutes at 4°C. Flow cytometric analysis was performed using a Cytomics FC500 flow cytometry system (Beckman-Coulter, Miami, FL).
Immunohistochemistry
Specimens were deparaffinized, rehydrated, and incubated with YM-1 monoclonal antibody at 23°C for 2 hours. Then, the specimens were incubated with biotin-conjugated secondary anti-rat IgG antibody (DakoCytomation, Glostrup, Denmark) for 30 minutes followed by incubation with peroxidase-conjugated biotin-streptavidin complex (Vectastain ABC kit; Vector Laboratories, Peterborough, UK) for 30 minutes. Color was developed with 3,3-diaminobenzidine tetrahydrochloride tablet sets (DakoCytomation).
Cell Proliferation Assays
The in vitro growth of stable transfectants was assessed using Cell Counting Kit-8 (Dojin Laboratories, Kumamoto, Japan). Briefly, 1 × 103 cells were seeded into a 96-well plate. The cells were allowed to grow for 1 to 4 days. Then, the cells were incubated with 10 μl of the water-soluble tetrazolium salt-8 reagent for 2 hours. The optical density was measured at 450 nm, with a 655-nm reference, using a microplate reader (model 550; Bio-Rad, Hercules, CA).
To examine three-dimensional proliferation of stable clones, 1 × 103 cells were seeded into a 96-well plate (Sumilon Celltight Spheroid; Sumilon, Tokyo, Japan). The spheroids were fed every other day by carefully replacing half of the spent medium with fresh medium. To calculate mean size of the spheroids, diameters were measured every other day. The cells were allowed to grow for 6 days.
In Vivo Detection of Platelets Associated with Aggrus-Expressing Cells and Cell Survival
The mock-transfected or WT-Aggrus-transfected CHO cells (CHO/control or CHO/WT-Aggrus, respectively) were stained with PKH67 using a green fluorescent cell linker kit (Sigma), according to the manufacturer’s instruction, and resuspended in Hanks’ balanced salt solution (HBSS) without calcium or magnesium to a final concentration of 2.5 × 106 cells/ml. Female BALB/c-nu/nu mice were injected intravenously with 200 μl of the labeled cells (5 × 105 cells/mouse), and lungs were removed 30 minutes and 6 hours after injection. The number of arrested cells was determined using fluorescence microscopy on 12 images at ×200 magnification from at least two mice at each time point per group. To detect association of CHO/WT-Aggrus cells with platelets, frozen mouse lung sections were stained 30 minutes after injection with an anti-mouse CD41 antibody (Becton Dickinson, San Jose, CA) followed by incubation with a biotinylated second antibody (DakoCytomation) and avidin R-phycoerythrin (Biomeda Corporation, Foster City, CA). The extent of platelet association with CHO/WT-Aggrus cells was quantitated by evaluating sections using conventional epifluorescence. Images were captured on a fluorescence microscopy at ×200 magnification. At least eight independent images for each lung were analyzed. In some experiments, mice were injected intraperitoneally with 75 mg/kg aspirin (Sigma) for 3 days until the day of intravenous injection of the stable clones (5 × 105 cells/mouse) into lateral tail vein of female BALB/c-nu/nu mice. Thirty minutes after cell injection, blood was drawn for testing platelet aggregation, and lungs were removed to count the arrested cells.
Experimental Lung Metastasis and Animal Survival
CHO/control, CHO/WT-Aggrus (clones 3 and 5), and CHO cells that were stably transfected with plasmid containing T34A-human Aggrus (CHO/T34A-Aggrus clones 4 and 5) or T52A-human Aggrus (CHO/T52A-Aggrus clones 10 and 48), and were harvested, washed, and resuspended in HBSS (2.5 × 106 cells/ml). Then, the stable clones (5 × 105 cells/mouse) were inoculated intravenously into lateral tail vein of female BALB/c-nu/nu mice. After 17 days, the mice were euthanized, and surface lung metastatic foci were counted and measured. The lung tissues from CHO/control and CHO/WT-Aggrus-bearing mice were processed for hematoxylin and eosin (H&E) and elastica-van Gieson (EVG) staining and immunohistochemical analysis to confirm the expression of human Aggrus in the metastatic foci. To investigate the effect of aspirin on metastasis formation, female BALB/c-nu/nu mice were injected intraperitoneally with aspirin (75 mg/kg) or phosphate-buffered saline (PBS) for 3 days until the day of intravenous injection of the stable clones (2.5 × 105 cells/mouse). Additional injections of aspirin or PBS were given daily until day 17. After 17 days of cell injection, lung foci were counted and measured. PBS was used as a control. For the survival study, the stable clones (5 × 105 cells/mouse) were inoculated intravenously into lateral tail vein of female BALB/c-nu/nu mice. The survival of the mice was checked daily for 50 days.
Tumorigenicity and Spontaneous Metastasis Assays
CHO/control and CHO/WT-Aggrus cells were harvested, washed, and resuspended in HBSS (5 × 107 cells/ml). Cells (5 × 106) in 0.1 ml of HBSS were injected subcutaneously into the back, close to the neck, of female BALB/c-nu/nu mice. Tumors were measured with calipers at 13, 15, 18, 20, 24, and 27 days after injection. Tumor volume was calculated by the following formula: volume = W2 × L/2, where W = short diameter and L = long diameter. Mice were euthanized 30 days after injection. Lungs and primary tumor tissues were harvested for H&E and EVG staining. Small portions of each tumor tissues were analyzed to confirm the human Aggrus expression by Western blot analysis.
Platelet Aggregation Assay
Platelet aggregation was monitored by measuring electric impedance29 using a whole-blood aggregometer (model 560; Chronolog, Havertown, PA). Heparinized blood was drawn from BALB/c mice or aspirin-pretreated BALB/c-nu/nu mice by cardiac puncture. Whole blood was then diluted with an equal amount of normal saline. The sample was placed in a plastic cuvette containing a magnetic stir bar and was kept at 37°C for 10 minutes before analysis. The platelet aggregation was then initiated by adding CHO/WT-Aggrus cells (5 × 106 cells/sample) or 10 μmol/L ADP (final concentration) and monitored for up to 20 minutes. In some experiments, whole blood was pretreated with appropriate concentrations of aspirin for 5 minutes at 37°C before stimulation.
Statistical Analysis
All data are shown as means ± SEM, except for cell proliferation assay data that is shown by means ± SD. Student’s t-test, Mann-Whitney U-test, and one-way analysis of variance followed by Tukey-Kramer multiple comparisons were performed, where appropriate. The mouse survival assay was evaluated by Kaplan-Meier analysis and the log rank test. P values less than 0.05 were considered statistically significant. All statistical tests were two-sided.
Results
Aggrus Induced Platelet Aggregation and Enhanced Cell Survival in Vivo
Our previous in vitro studies suggested that Aggrus was associated with TCIPA.15 To confirm further the role of Aggrus in in vivo TCIPA and metastasis formation, we injected intravenously green fluorescent (PKH67)-labeled CHO/control or CHO/WT-Aggrus cells into mice and analyzed their pulmonary retention. Several reports suggested that TCIPA was observed 30 minutes after tumor cell injection.30,31 Thus, we examined the pulmonary retention of the Aggrus-expressing cells after 30 minutes and 6 hours of cell injection. The number of PKH67-labeled CHO/WT-Aggrus cells retained in the lung microvasculature was significantly higher than that of CHO/control cells (Figure 1B, P < 0.001). Pulmonary retention of CHO/WT-Aggrus cells, but not CHO/control cells, was also observed 6 hours after injection (Figure 1, A and B). Thus, Aggrus could be associated with the initial arrest and the survival of CHO/WT-Aggrus cells in vivo. To detect Aggrus-induced platelet aggregation in vivo, frozen lung sections were stained with an antibody to CD41, a defined marker for platelets in mouse.30,31 At 30 minutes after cell injection, extensive platelet decoration (red) around CHO/WT-Aggrus cells (green) was observed in 38.8% of the cells, whereas only 7.6% of CHO/control cells were covered with platelets (Figure 1, C and D; P < 0.05). These findings suggest that Aggrus facilitates initial arrest in the lung microvasculature by inducing platelet aggregation and contributes to initial survival of the tumor cells to promote metastasis formation.
Figure 1.
Survival of Aggrus-expressing cells and its interactions with platelets in vivo. Nude mice were injected intravenously with the fluorescent dye PKH67-labeled CHO/control (control) or CHO/WT-Aggrus clone 5 (WT). Mice were euthanized 30 minutes and 6 hours after injection. Frozen sections of the lungs were resected, and the green-colored cells were counted. A: Representative images of the PKH67-labeled cells that were arrested in lung 6 hours after cell injection. B: The number of green-colored cells in lung sections. At least 12 independent images for each lung were analyzed. The number of foci in CHO/WT-Aggrus-injected mice was statistically significant both 30 minutes and 6 hours. *P < 0.001 by analysis of variance with the Tukey-Kramer test. C: The in vivo interaction between platelets and intravenously injected PKH67-labeled cells after 30 minutes of inoculation. Frozen sections of the lungs were stained with an anti-CD41 antibody for platelets. The PKH67-labeled cells were observed in green, and platelets were observed in red. D: The numbers of PKH67-labeled cells covered with platelets were quantitated by evaluating the sections. **P < 0.05 by Mann-Whitney U-test. Scale bars: 100 μm (A); 10 μm (C). Original magnifications, ×200.
Threonine-34 (T34) and Threonine-52 (T52) in Human Aggrus Are Critical Sites for Platelet Aggregation-Inducing Ability
We previously showed that human Aggrus had one PLAG domain containing T52 (in the PLAG3 domain), that mouse Aggrus had one domain containing T34 (in the PLAG1 domain), and that these threonine residues were critical for their platelet aggregation-inducing activities.15 Detailed alignment of the Aggrus homologues revealed that Aggrus contained three tandem repeats of the PLAG domains (PLAG1 to 3) and that the threonine residues in the PLAG1 and the PLAG3 seem to be critical for the activity.27 However, the significance of the T34 in the PLAG1 domain of human Aggrus has not been determined yet. Therefore, we confirmed the importance of T34 in platelet-aggregating activity of human Aggrus by establishing stable transfectants. We established stable CHO clones expressing WT human Aggrus (CHO/WT-Aggrus clones 3 and 5; WT-3 and WT-5), human Aggrus containing a T34A mutation (CHO/T34A-Aggrus clones 4 and 5; T34A-4 and -5), human Aggrus containing a T52A mutation (CHO/T52A-Aggrus clones 10 and 48; T52A-10 and -48), and vector alone (CHO/control; control). Western blot analysis showed all clones, with the exception of the CHO/control, expressed Aggrus protein (Figure 2A). Flow cytometric analysis suggested that WT and point-mutated Aggrus proteins were trafficking to the cell surface properly (Figure 2B). The stable clones grew at similar rate throughout 72 hours (Figure 2C), suggesting that Aggrus expression did not affect CHO cell growth in vitro. To examine anchorage-independent growth of the clones, three-dimensional spheroid growth was compared. Spheroids were measured for 6 days. We could not see any difference in anchorage-independent growth (Figure 2D). These cells were further tested for their ability to induce platelet aggregation using whole blood aggregometer. Both CHO/WT-Aggrus clones (WT-3 and WT-5) induced platelet aggregation (Figure 2E). In contrast, no platelet aggregation could be observed when CHO/control, CHO/T34A-Aggrus (T34A-4 and T34A-5), or CHO/T52A-Aggrus (T52A-10 and T52A-48) was incubated with whole blood (Figure 2E). These results indicate that the T34 residue in the PLAG1 domain, in addition to the T52 in the PLAG3 domain,15 are essential sites for exhibiting platelet aggregation-inducing ability of human Aggrus.
Figure 2.
Characterization of WT-Aggrus-expressing CHO clone 3 and clone 5 (WT-3 and WT-5), platelet aggregation-deficient Aggrus mutant-expressing CHO clones (T34A-4, T34A-5, T52A-10, and T52A-48), or mock-transfected CHO clone (control). A: Cell lysates were electrophoresed and immunoblotted with an anti-human Aggrus antibody (top) or an anti-β-actin antibody (bottom). B: Flow cytometric analysis to confirm the cell surface expression of Aggrus. Cells were treated with an anti-human Aggrus antibody, and then cells were incubated with Oregon Green 488-conjugated secondary antibody. C and D: Growth of CHO clones in monolayer culture (C) or in three-dimensional spheroid culture (D). Data are means ± SD of triplicate determinations. E: Platelet aggregation-inducing ability of each clone was estimated by incubating with the diluted mouse whole blood, as described in Materials and Methods.
Aggrus Promotes Pulmonary Metastasis in a Platelet Aggregation-Dependent Manner
To address the function of Aggrus in vivo, we inoculated intravenously the stable Aggrus transfectants into female BALB/c-nu/nu mice. Injection of CHO/WT-Aggrus led to development of multiple lung metastatic foci (Figure 3A). Pulmonary metastasis was rarely observed in mice injected with CHO/control, platelet aggregation-deficient CHO/T34A-Aggrus, or CHO/T52A-Aggrus (Figure 3A). The median number of pulmonary metastatic foci (range) was 4.9 (0 to 21) in control, 151 (34 to 235) in WT-3, 209 (150 to 266) in WT-5, 3 (0 to 18) in T34A-4, 19 (2 to 49) in T34A-5, 2.7 (0 to 12) in T52A-10, and 3.2 (0 to 16) in T52A-48 (Figure 3B). The number of metastatic lung nodules in mice injected with CHO/control, CHO/T34AAggrus, or CHO/T52A-Aggrus clones was significantly lower than that in mice injected with CHO/WT-Aggrus clones (Figure 3B, P < 0.001). Consistent with the increase in the number of metastatic foci, lung weight was higher in CHO/WT-Aggrus-injected mice than in mice injected with CHO/control, CHO/T34A-Aggrus, or CHO/T52A-Aggrus (Figure 3C). We did not observe, macroscopically, metastatic foci in the liver, kidney, spleen, colon, or ovary in any of the mice (data not shown).
Figure 3.
The expression of WT-Aggrus, but not platelet aggregation-deficient Aggrus mutants (T34A and T52A), promotes pulmonary metastasis in vivo. Nude mice were euthanized 17 days after intravenous injection of CHO/control (control, n = 9), CHO/WT-Aggrus clone 3 (WT-3, n = 6), CHO/WT-Aggrus clone 5 (WT-5, n = 9), CHO/T34A-Aggrus clone 4 (T34A-4, n = 9), CHO/T34A-Aggrus clone 5 (T34A-5, n = 6), CHO/T52A-Aggrus clone 10 (T52A-10, n = 7), and CHO/T52A-Aggrus clone 48 (T52A-48, n = 6). A: Representative pictures of the lungs of control, WT-5 (WT), T34A-4 (T34A), and T52A-10 (T52A) are shown. B and C: Detailed numbers of the lung metastasis nodules (B) and weight of the lung (C) in each mouse are shown. B: Bars indicate mean value. C: Data are means ± SEM. *P < 0.001, compared with control; +P < 0.001, compared with T34A-4; #P < 0.001, compared with T34A-5; −P < 0.001, compared with T52A-10 or ‡P < 0.001, compared with T52A-48; **P < 0.05, compared with control; and ##P < 0.05, compared with T34A-4 by analysis of variance with Tukey-Kramer test.
Aspirin is known to attenuate metastasis formation by inhibiting platelet aggregation.32,33 To confirm that the platelet aggregation was directly associated with Aggrus-mediated metastasis, we examined the effects of aspirin on Aggrus-mediated pulmonary metastasis. When platelets were pretreated with aspirin in vitro (0 to 100 μmol/L) for 5 minutes before analysis, Aggrus-induced platelet aggregation was inhibited in a concentration-dependent manner (Figure 4A). We then examined aggregating ability of platelets that were prepared from aspirin-treated mice. The mice were administered with aspirin for 3 days until the day of platelet preparation. As shown in Figure 4B, in vivo aspirin-treated platelets could not aggregate even when platelet aggregation was induced by 10 μmol/L ADP. In contrast, PBS administration did not affect the platelet-aggregating ability.
Figure 4.
Aspirin suppressed Aggrus-mediated platelet aggregation in vitro and metastasis formation in vivo. A: Diluted whole blood was incubated with the indicated concentrations of aspirin for 5 minutes. The platelet aggregation was then initiated by adding CHO/WT-Aggrus clone 5. B and C: Nude mice were intraperitoneally administered aspirin or PBS for 3 days, as described in Materials and Methods. B: The platelet aggregation was then initiated by adding 10 μmol/L ADP. C: Frozen sections of the lungs were resected after 30 minutes of intravenous injection of CHO/control (control) or CHO/WT-Aggrus clone 5 (WT), and the arrested cells were counted. At least eight images for each lung were analyzed. *P < 0.0001, by analysis of variance with Tukey-Kramer test. D and E: Nude mice were injected intraperitoneally with aspirin or PBS for 3 days until the day of the intravenous injection of CHO/WT-Aggrus clone 5. After cell inoculation, mice were further administered aspirin or PBS daily for 17 days. Number of lung nodules (D) and lung weight (E) in each mouse were analyzed after 17 days of injection. Data are means ± SEM. **P < 0.05, by Mann-Whitney U-test.
Detailed analysis of lung sections revealed that pulmonary retention of CHO/WT-Aggrus cells was inhibited by aspirin administration (Figure 4C, P < 0.0001). Consistent with the results, treatment of the mice with aspirin suppressed metastasis formation of CHO/WT-Aggrus cells (Figure 4D). Lung weight of aspirin-treated mice was significantly lighter than that of PBS-treated mice (Figure 4E, P < 0.05). These results demonstrate that platelet aggregation was directly associated with the Aggrus-induced pulmonary metastasis.
Histological analysis of the lung tissues from CHO/WT-Aggrus-injected mice shows the typical features of the metastatic tumors. First, metastatic tumors were detected in the lungs of the CHO/WT-Aggrus-injected mouse. Metastatic tumors and the tumor embolus, occupying and attaching to the pulmonary arteriole (indicated by arrowheads in Figure 5B), are sequential. The lungs from mice that were injected with CHO/control showed no metastatic lesions (Figure 5A). Second, EVG staining clarified that the venule was totally occluded by the tumor embolus of the CHO/WT-Aggrus-expressing cells (Figure 5C). Third, Aggrus staining was observed in the metastatic lesions when the lung tissues were stained with our previously generated YM-1 monoclonal antibody that could specifically recognize human Aggrus but not mouse Aggrus.24,25,28 At high-power magnification, drifted tumor cell clump in the venule showed strong membranous staining (Figure 5D, inset). In addition, the Aggrus-expressing cells made a mass with platelets, leukocytes, and erythrocytes (Figure 5D, arrow, inset). Aggrus-expressing cells also attached to the endothelial cells (Figure 5D, asterisk, inset). These attachments of tumor cells to both leukocytes and endothelial cells are suggested to promote extravasation in the metastatic cascade.34
Figure 5.
Formation of emboli in CHO/WT-Aggrus-formed metastatic tumors. Nude mice were euthanized 17 days after intravenous injection of CHO/control or CHO/WT-Aggrus clone 5. Tumor specimens of the lungs were subjected to histochemical analysis. A and B: H&E staining shows CHO/WT-Aggrus cells, but not CHO/control cells, formed metastatic tumors. Embolized tumor cells, shown by an asterisk, were seen in the pulmonary arteriole (B: arrowheads, pulmonary arteriole; Br., bronchi), whereas CHO/control cells rarely formed metastasis (A). C: The lumen of the venule (stained by EVG, arrowheads) was occluded by CHO/WT-Aggrus-formed tumor embolus. D: Expression of Aggrus in the lesions was confirmed by immunohistochemical studies. Aggrus expression was observed in the lungs of mice injected with CHO/WT-Aggrus cells. Aggregates of circulating Aggrus-expressing tumor cell clump with platelets, erythrocytes, and leukocytes were detected in the venule (D: inset, arrow). The inset shows magnification of areas of interest. Attachment of Aggrus-expressing cells to endothelial cells are shown by an asterisk. T, tumor cells. Scale bars = 100 μm.
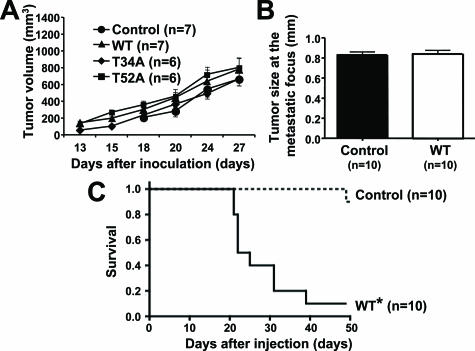
There were no differences in the in vivo growth of WT or point-mutated Aggrus-expressing clones when these clones were inoculated subcutaneously (Figure 6A). Because pulmonary metastasis was slightly observed in mice injected with CHO/control (Figure 3B), we compared the tumor size at the metastatic nodules of CHO/control and CHO/WT-Aggrus clones. As shown in Figure 6B, the size of nodules was almost the same. These results suggest that Aggrus expression does not contribute to the in vivo growth of CHO cells. Therefore, Aggrus may promote the formation of pulmonary metastasis via its platelet aggregation-inducing activity without affecting the growth potential.
Figure 6.
Aggrus expression does not confer in vivo CHO cell growth but decreases mouse survival. A: CHO/control (control), CHO/WT-Aggrus clone 5 (WT), CHO/T34A-Aggrus clone 4 (T34A), and CHO/T52A-Aggrus clone 10 (T52A) were inoculated subcutaneously into nude mice. Tumor diameter was measured with calipers at 13, 15, 18, 20, 24, and 27 days after injection, as described in Materials and Methods. Data are means ± SEM. B: Surface lung tumor size in the nude mice injected with CHO/control (control) or CHO/WT-Aggrus clone 5 (WT) was measured. Data are means ± SEM. C: Survival rate of mice bearing CHO/control (control) or CHO/WT-Aggrus clone 5 (WT) throughout time. *P < 0.0001 versus control evaluated by Kaplan-Meier analysis and the log rank test.
We also evaluated the survival of mice after intravenous injection of CHO/control or CHO/WT-Aggrus clones. We observed significantly poorer survival rates of mice injected with CHO/WT-Aggrus cells than those of mice injected with CHO/control (Figure 6C, P < 0.0001). All mice in the CHO/control group survived until 40 days of cell injection, whereas 9 of the 10 mice (90%) in CHO/WT-Aggrus group died from cachexia with a large number of lung metastatic nodules within 40 days. One of the ten CHO/control-injected mice died on day 49, accompanied by forming two large lung nodules (data not shown). These results suggest that Aggrus expression contributes to metastatic tumor formation in the lung and decreases survival of the mice.
Aggrus Expression Promotes Spontaneous Metastasis
As shown in Figure 3, Aggrus could promote the formation of pulmonary metastasis in the experimental metastasis model. To confirm this metastasis-promoting potential, we next examined whether CHO/WT-Aggrus could form pulmonary metastasis when these cells were inoculated subcutaneously into nude mice. Mice were euthanized at day 30. The lungs were examined for metastases by H&E staining, and the pulmonary blood vessels were visualized by EVG staining. CHO/WT-Aggrus cells formed metastatic nodules in the lung, whereas CHO/control cells showed much fewer or no metastatic lesions (Figure 7A, left and middle). A significant increase in the number of metastatic foci in the lung and the lung weight was seen in mice injected with CHO/WT-Aggrus, when compared with those in mice injected with CHO/control (Figure 7, B and C). Examination of all specimens revealed that six of seven mice (86%) injected with CHO/WT-Aggrus formed pulmonary micrometastases, whereas only one of seven (14%) control mice did. Consistent with the experimental metastasis model, tumor embolus within the pulmonary arteriole was observed and was attached to the metastatic lesions (Figure 7A, right). There was no difference in tumor volume at the primary sites between these two groups (Figure 6A). Moreover, morphology of the primary tumors seemed not to be different between the two groups (data not shown). Western blot analysis revealed that Aggrus expression in the primary tumors of CHO/WT-Aggrus cells continued 30 days after injection (Figure 7D). These results indicate that Aggrus acts as a metastasis-promoting factor without affecting tumor cell growth.
Figure 7.
Aggrus expression promotes spontaneous metastasis. A: Nude mice were injected subcutaneously with CHO/control (control, left) or CHO/WT-Aggrus clone 5 (WT, middle and right) cells. Mice were euthanized at day 30, and metastases in the lungs were examined by H&E staining (left and middle). EVG staining was used to detect pulmonary blood vessels (right). T, tumor; arrowheads, pulmonary arteriole. The arteriole was filled with tumors. B and C: The numbers of micrometastases in the lungs (B) and the lung weight (C) were determined. Data are means ± SEM. *P < 0.01, and **P < 0.05 by Mann-Whitney U-test. D: The lysates of CHO/control (control, left) or CHO/WT-Aggrus clone 5 (WT, right) xenografts were electrophoresed and immunoblotted with an anti-Aggrus antibody (top) or an anti-β-actin antibody (bottom). Scale bars = 100 μm. Original magnifications: ×100 (A, left and middle); ×400 (A, right).
Discussion
Platelets have long been suspected of having a role in cancer progression and metastasis.35 We previously identified human Aggrus/podoplanin as a platelet aggregation-inducing factor that was expressed on the surface of various types of tumor cells.15 Because CHO cells expressed very low level of Aggrus homologue and several CHO mutants that display inability in distinct steps of glycosylation (eg, Lec series) were available for the analysis of O-glycans attached to Aggrus, we used CHO for the analysis of Aggrus function. Although ectopic expression of WT human Aggrus on CHO cell surface could induce platelet aggregation in vitro,15 its role in metastasis formation in vivo was still unknown. Thus, we examined whether stable Aggrus-transfected CHO cells could form pulmonary metastasis in experimental and spontaneous metastasis models. In the experimental metastasis model, increase in the number of pulmonary metastatic nodules was observed by WT-Aggrus expression (Figure 3). In addition, in vivo interaction between WT-Aggrus-expressing cells and platelets could be detected within 30 minutes of infusion (Figure 1). Furthermore, the numbers of WT-Aggrus-expressing cells initially arrested in the lung were higher than those of control cells (Figure 1B). These findings indicate that Aggrus induced platelet aggregation soon after the cell injections, and that might help the survival of the cells and formed metastasis. Aggrus expression also promoted spontaneous lung metastasis (Figure 7) without affecting the growth at the primary site (Figure 6A). This finding was supported by the fact that the size of metastatic foci in the experimental model was not affected by Aggrus expression (Figure 6B). In agreement with the above results, the survival rate in the mice bearing WT-Aggrus-expressing cells was significantly lower than that of the mice carrying control cells (Figure 6C). These findings suggest that Aggrus could be a potential molecular marker to estimate the possibility to form metastasis and could portend a poor prognosis.
We also clarified previously that the sialylated O-glycan of human Aggrus was critical for its platelet aggregation-inducing activity using the glycosylation-deficient CHO cell lines.28 We identified three tandem repeats of the segment of EDxxVTPG in the extracellular domain as the PLAG domain, which is critical for the platelet-aggregating activity of Aggrus.27 In human Aggrus, we here confirmed the importance of the threonine-34 (T34) in the PLAG1 domain, in addition to the threonine-52 (T52) in the PLAG3 domain, by analyzing the platelet aggregation-inducing activity of their point mutants T34A or T52A-Aggrus (Figure 2). Because threonine followed by proline is likely to be O-glycosylated36 and Edman degenerative microsequencing of the equivalent residues in the dog homologue of Aggrus revealed gaps at the threonine residues,37 sialylated O-glycan attached to the T34 and the T52 in human Aggrus might be associated with its platelet aggregation-inducing capability. Using the mutants, we performed the experimental metastasis assay and found that the platelet aggregation-deficient T34A or T52A-Aggrus lacked the metastasis-forming activity (Figure 3). Therefore, the platelet-aggregating activity is directly involved in Aggrus-derived metastasis formation.
To confirm further that role of platelets in the Aggrus-mediated pulmonary metastasis in vivo, we examined the effect of aspirin as an inhibitor of platelet aggregation. Gasic and colleagues32,33 and Kolenich and colleagues38 reported that the aspirin administration into mice decreased the frequency of lung metastases. Aspirin inhibited Aggrus-induced platelet aggregation in vitro (Figure 4A). Because the ADP-induced aggregation of platelets was suppressed in aspirin-administered mice (Figure 4B), aspirin might inhibit Aggrus-mediated platelet aggregation in vivo. As expected, pulmonary arrest of CHO/WT-Aggrus cells was significantly lower in mice pretreated with aspirin than that in PBS-treated mice (Figure 4C). Moreover, aspirin administration decreased the formation of experimental metastasis (Figure 4, D and E). These results strongly indicate that platelet aggregation is indispensable for the Aggrus-mediated pulmonary metastasis.
With careful examination of the lung specimens derived from both experimental and spontaneous metastasis models, we observed that the metastatic lesions were attached to the tumor emboli within the pulmonary arterioles (Figure 5B and Figure 7A). Drifting tumor cell clumps, which expressed Aggrus, were found in the venule (Figure 5D). These tumor cell clumps seem to have higher metastatic efficiencies than single cells.34 In addition, attachment of Aggrus-expressing cells to both leukocytes and endothelial cells, which could be facilitated by platelets,39 was also observed. This step was reported to promote the essential step of arrest in the capillary5 and extravasation in the metastatic cascade.39 We speculate that Aggrus-induced platelet aggregation might facilitate the survival of the tumor cells by protecting against immune systems, promoting proliferation within the vasculature using platelet-releasing growth factors, forming tumor emboli, and expanding continuously to the extravascular lung tissues that resulted in the formation of metastatic foci.
Aggrus expression could also be detected in highly metastatic B16 melanoma variant, B16-F10,40 and in highly metastatic mouse colon adenocarcinoma 26 variant, NL-17.41 Our previously established anti-mouse Aggrus antibody 8F11 inhibited platelet aggregation induced by both cell lines.40,41 Moreover, the antibody suppressed pulmonary metastasis of NL-17 cells.42 These results suggest that the present data are not unique to CHO cell line.
In conclusion, the present study shows that Aggrus promoted experimental and spontaneous metastasis, without affecting tumor growth, and diminished survival of mice. Pulmonary metastasis was suppressed by abrogation of its platelet-aggregating activity. Our studies provide clear evidence for a causative role of platelet aggregation in cancer metastasis. Based on these studies, we suggest that small molecule inhibitors or antibodies against human Aggrus could be a therapeutic strategy for inhibiting tumor metastasis and for enhancing cancer patient survival.
Acknowledgments
We thank Drs. Kazuo Harada, Yoshinao Kikuchi, and Mika Kato Kaneko for the critical reading and invaluable comments on the manuscript; Dr. Takao Shimizu for the aggregometer; Shigeo Sato and Yasuyuki Kihara for their excellent technical advice on the animal experiments; and Chizuko Hashimoto for technical support with immunocytochemistry.
Footnotes
Address reprint requests to Naoya Fujita, Ph.D., Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, 3-10-6, Ariake, Koto-ku, Tokyo 135-8550, Japan. E-mail: naoya.fujita@jfcr.or.jp.
Supported in part by the Ministry of Education, Culture, Sports, Science, and Technology, Japan (special grants 17016012 and 18390020 to T.T. and N.F.); the Japanese Society for the Promotion of Science for Young Scientists, Japan (grant for research fellowships to A.K.); the Kato Memorial Bioscience Foundation, Japan (to N.F.); and the Takeda Science Foundation, Japan (to N.F.).
References
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- Philippe C, Philippe B, Fouqueray B, Perez J, Lebret M, Baud L. Protection from tumor necrosis factor-mediated cytolysis by platelets. Am J Pathol. 1993;143:1713–1723. [PMC free article] [PubMed] [Google Scholar]
- Malik AB. Pulmonary microembolism. Physiol Rev. 1983;63:1114–1207. doi: 10.1152/physrev.1983.63.3.1114. [DOI] [PubMed] [Google Scholar]
- Mehta P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood. 1984;63:55–63. [PubMed] [Google Scholar]
- Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- Verheul HM, Hoekman K, Lupu F, Broxterman HJ, van der Valk P, Kakkar AK, Pinedo HM. Platelet and coagulation activation with vascular endothelial growth factor generation in soft tissue sarcomas. Clin Cancer Res. 2000;6:166–171. [PubMed] [Google Scholar]
- Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res. 1999;5:487–491. [PubMed] [Google Scholar]
- Salgado R, Vermeulen PB, Benoy I, Weytjens R, Huget P, Van Marck E, Dirix LY. Platelet number and interleukin-6 correlate with VEGF but not with bFGF serum levels of advanced cancer patients. Br J Cancer. 1999;80:892–897. doi: 10.1038/sj.bjc.6690437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierodzik ML, Plotkin A, Kajumo F, Karpatkin S. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. J Clin Invest. 1991;87:229–236. doi: 10.1172/JCI114976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi N, Fan D, Fidler IJ. Inhibition of murine melanoma experimental metastasis by recombinant desulfatohirudin, a highly specific thrombin inhibitor. Cancer Res. 1991;51:4549–4556. [PubMed] [Google Scholar]
- Camez A, Dupuy E, Bellucci S, Calvo F, Bryckaert MC, Tobelem G. Human platelet-tumor cell interactions vary with the tumor cell lines. Invasion Metastasis. 1986;6:321–334. [PubMed] [Google Scholar]
- Honn KV, Steinert BW, Moin K, Onoda JM, Taylor JD, Sloane BF. The role of platelet cyclooxygenase and lipoxygenase pathways in tumor cell induced platelet aggregation. Biochem Biophys Res Commun. 1987;145:384–389. doi: 10.1016/0006-291x(87)91333-7. [DOI] [PubMed] [Google Scholar]
- Jurasz P, Sawicki G, Duszyk M, Sawicka J, Miranda C, Mayers I, Radomski MW. Matrix metalloproteinase 2 in tumor cell-induced platelet aggregation: regulation by nitric oxide. Cancer Res. 2001;61:376–382. [PubMed] [Google Scholar]
- Kato Y, Fujita N, Kunita A, Sato S, Kaneko M, Osawa M, Tsuruo T. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- Martín-Villar E, Scholl FG, Gamallo C, Yurrita MM, Munoz-Guerra M, Cruces J, Quintanilla M. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo T, Osawa M. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumour Biol. 2005;26:195–200. doi: 10.1159/000086952. [DOI] [PubMed] [Google Scholar]
- Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, Mao L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563–569. doi: 10.1002/cncr.22061. [DOI] [PubMed] [Google Scholar]
- Kimura N, Kimura I. Podoplanin as a marker for mesothelioma. Pathol Int. 2005;55:83–86. doi: 10.1111/j.1440-1827.2005.01791.x. [DOI] [PubMed] [Google Scholar]
- Kato Y, Sasagawa I, Kaneko M, Osawa M, Fujita N, Tsuruo T. Aggrus: a diagnostic marker that distinguishes seminoma from embryonal carcinoma in testicular germ cell tumors. Oncogene. 2004;23:8552–8556. doi: 10.1038/sj.onc.1207869. [DOI] [PubMed] [Google Scholar]
- Shibahara J, Kashima T, Kikuchi Y, Kunita A, Fukayama M. Podoplanin is expressed in subsets of tumors of the central nervous system. Virchows Arch. 2006;448:493–499. doi: 10.1007/s00428-005-0133-x. [DOI] [PubMed] [Google Scholar]
- Mishima K, Kato Y, Kaneko MK, Nishikawa R, Hirose T, Matsutani M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006;111:483–488. doi: 10.1007/s00401-006-0063-y. [DOI] [PubMed] [Google Scholar]
- Mishima K, Kato Y, Kaneko MK, Nakazawa Y, Kunita A, Fujita N, Tsuruo T, Nishikawa R, Hirose T, Matsutani M. Podoplanin expression in primary central nervous system germ cell tumors: a useful histological marker for the diagnosis of germinoma. Acta Neuropathol. 2006;111:563–568. doi: 10.1007/s00401-006-0033-4. [DOI] [PubMed] [Google Scholar]
- Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kaneko MK, Kato Y, Kitano T, Osawa M. Conservation of a platelet activating domain of Aggrus/podoplanin as a platelet aggregation-inducing factor. Gene. 2006;378:52–57. doi: 10.1016/j.gene.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Kato Y, Kunita A, Fujita N, Tsuruo T, Osawa M. Functional sialylated O-glycan to platelet aggregation on Aggrus (T1alpha/podoplanin) molecules expressed in Chinese hamster ovary cells. J Biol Chem. 2004;279:38838–38843. doi: 10.1074/jbc.M407210200. [DOI] [PubMed] [Google Scholar]
- Cardinal DC, Flower RJ. The electronic aggregometer: a novel device for assessing platelet behavior in blood. J Pharmacol Methods. 1980;3:135–158. doi: 10.1016/0160-5402(80)90024-8. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95:9325–9330. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic GJ, Gasic TB, Murphy S. Anti-metastatic effect of aspirin. Lancet. 1972;2:932–933. doi: 10.1016/s0140-6736(72)92581-0. [DOI] [PubMed] [Google Scholar]
- Gasic GJ, Gasic TB, Galanti N, Johnson T, Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973;11:704–718. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–351. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Suzuki M, Ikenaga H, Takeuchi M. Discovery of the shortest sequence motif for high level mucin-type O-glycosylation. J Biol Chem. 1997;272:16884–16888. doi: 10.1074/jbc.272.27.16884. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Lottspeich F, Maisner A, Klenk HD, Herrler G. Molecular characterization of gp40, a mucin-type glycoprotein from the apical plasma membrane of Madin-Darby canine kidney cells (type I). Biochem J. 1997;326:99–108. doi: 10.1042/bj3260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenich JJ, Mansour EG, Flynn A. Haematological effects of aspirin. Lancet. 1972;2:714. doi: 10.1016/s0140-6736(72)92124-1. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973;9:223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sugimoto Y, Tsuruo T. Expression of a Mr 41,000 glycoprotein associated with thrombin-independent platelet aggregation in high metastatic variants of murine B16 melanoma. Cancer Res. 1990;50:6657–6662. [PubMed] [Google Scholar]
- Watanabe M, Okochi E, Sugimoto Y, Tsuruo T. Identification of a platelet-aggregating factor of murine colon adenocarcinoma 26: Mr 44,000 membrane protein as determined by monoclonal antibodies. Cancer Res. 1988;48:6411–6416. [PubMed] [Google Scholar]
- Sugimoto Y, Watanabe M, Oh-hara T, Sato S, Isoe T, Tsuruo T. Suppression of experimental lung colonization of a metastatic variant of murine colon adenocarcinoma 26 by a monoclonal antibody 8F11 inhibiting tumor cell-induced platelet aggregation. Cancer Res. 1991;51:921–925. [PubMed] [Google Scholar]