Abstract
The mechanisms underlying the hypoxia-induced disruption of the barrier function of neural vasculature were analyzed with reference to the expression of claudin-5, a component of tight junctions between neural endothelial cells. The movement of claudin-5 from the cytoplasm to the plasma membrane of cultured confluent brain-derived endothelial (bEND.3) cells was closely correlated with the increase in the transendothelial electrical resistance. Inhibition of the expression of claudin-5 by RNAi resulted in a reduction of transendothelial electrical resistance, indicating a critical role of claudin-5 in the barrier property. Hypoxia (1% O2) altered the location of claudin-5 in the plasma membrane and the level of claudin-5 protein in bEND.3 cells, and these changes were accompanied by a decrease in the transendothelial electrical resistance. In vivo the claudin-5 molecules were expressed under normoxia in the plasma membrane of retinal microvascular endothelial cells but were significantly reduced under hypoxic conditions. Tracer experiments revealed that the barrier function of hypoxic retinal vasculature with depressed claudin-5 expression was selectively disrupted against small molecules, which is very similar to the phenotype of claudin-5-deficient mice. These in vitro and in vivo data indicate that claudin-5 is a target molecule of hypoxia leading to the disruption of the barrier function of neural vasculature.
Homeostasis of the microenvironment is essential for the normal functioning of the nervous system, and it is maintained in part by the blood-brain barrier and blood-retinal barrier. These barriers are formed by the endothelial cells of neural tissue-specific vasculature. The barrier properties of vascular endothelial cells in the nervous system is not intrinsic to the endothelial cells of neural tissue but is established during embryonic development under influence of the tissues surrounding the vessels.1,2,3,4,5,6 It has been shown that the barrier properties can be induced in developing blood vessels of nonneural tissue if they are grown in a neural environment.1,6 In adults, the barrier function of the neural vasculature is not static and is regulated dynamically in response to changes in the surrounding environment, eg, changes in tissue oxygen concentration7,8,9 or by inflammatory processes.10,11 In fact, the blood-brain barrier and blood-retinal barrier are known to be disrupted in pathological situations such as in cerebral ischemic diseases and diabetic retinopathy, and the breakdown can lead to disturbances of the tissue microenvironment, which subsequently accelerates the progression of disease processes.7,12
The barrier properties of neural blood vessels are attributed mainly to the presence of complex tight junction networks between the endothelial cells.13 The identification of the integral membrane molecules of tight junctions was a turning point in the research on the molecular basis of tight junctions.14,15,16 Occludin was the first identified membrane component of tight junctions,17 but subsequent studies including gene knockout analyses demonstrated that occludin was not essential for the tight junction assembly and rather was involved in the signal transduction of endothelial cells.18 Later, the claudins were found to be other components of tight junctions,19 and accumulative evidence has revealed that they are the key molecules in the tight junction assembly.14,15,16,20,21 The claudins are a multigene family of more than 20 members. The pattern of expression of the different claudin family members varies among the tissue types, which confers the tissue-specific properties to the tight junctions.14 The claudins expressed in the endothelial cells of neural tissue are claudin-1, claudin-3, claudin-5, and claudin-12,22,23,24 and they are suggested to be the candidate molecules responsible for endothelial barrier function. A study of claudin-5-deficient mice disclosed that claudin-5 is indispensable for the barrier function of neural blood vessels for small molecules.23
Tissue oxygen concentration is known to influence the expression of various molecules including growth factors, cytokines, and enzymes.25 Hypoxia stimulates the cellular production of erythropoietin,26 vascular endothelial growth factor,27 tyrosine hydroxylase,28 phosphoglycerate kinase 1, and lactate dehydrogenase A.29 Many of these hypoxia-sensitive molecules are implicated in the adaptive processes of organisms to hypoxic circumstances, such as erythropoiesis, angiogenesis, increase in respiratory volume, and conversion of the metabolism to an anaerobic state. In addition, changes in the expression of certain oxygen-sensitive molecules can also trigger the progression of disease processes in patients with cerebral ischemic diseases, diabetic retinopathy, and other diseases.30
Despite recent advancements in the research on tight junctions, little is known about how the neural vascular endothelial cells lose their barrier properties under hypoxic conditions. Among the components of tight junctions, occludin, claudin-1, and claudin-3 are reported to be hypoxia-sensitive molecules,9,25,31 suggesting that the tight junction structure is hypoxia-sensitive. Here, we demonstrate that the expression and location of claudin-5 in neural microvascular endothelial cells is altered by tissue hypoxia resulting in a breakdown of the neural endothelial barrier.
Materials and Methods
Cell Culture
A mouse brain endothelial cell line, bEND.3, was obtained from the American Type Culture Collection (Manassas, VA) and cultured in fibronectin-coated culture dishes (BD Biosciences, Franklin Lakes, NJ) or on fibronectin-coated cell inserts with 0.4-μm pore size (BD Biosciences). The culture medium was Dulbecco’s modified Eagle’s medium (4500 mg/L glucose) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 10 μg/ml streptomycin. The cells were incubated with a CO2 level of 5% either with 20% O2 (atmospheric air) for normoxia or with 1% O2 balanced with N2 for hypoxia. The hypoxic condition was generated in an oxygen-regulated incubator (Personal Multi Gas Incubator; Astec, Tokyo, Japan).
Animal Studies
All animal experiments adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Laboratory Animal Care and Use Committee at the School of Medicine, Keio University. Adult male C57BL/6J mice (7 to 10 weeks old; CLEA Japan, Tokyo, Japan) were maintained in a chamber in which the O2 concentration could be regulated by controlling the inflow rates of O2 and N2. For hypoxic condition, the O2 concentration was maintained at 7 to 9% as described,32 and the oxygen concentration was continuously monitored with an oxygen analyzer (Max O2; Maxtec, Salt Lake City, UT). After 7 days, the eyes were enucleated immediately after removing the animals from the chambers, and the retinas were isolated for immunohistochemistry or Western blotting. For immunohistochemistry, flat mounts of the retinas were prepared by removing the cornea, lens, sclera, and vitreous from eyes, which had been briefly fixed in 4% paraformaldehyde.
Western Blotting
Proteins were extracted from cultured bEND.3 cells and from isolated retinas by incubating them in phosphate-buffered saline (PBS) containing 10% Triton X-100, 0.2% sodium dodecyl sulfate, 1 mmol/L sodium vanadate, 10 mmol/L sodium fluoride, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, and 1 μg/ml pepstatin. The proteins were separated on 12.5% sodium dodecyl sulfate-polyacrylamide by gel electrophoresis under reducing conditions and were electrophoretically transferred onto polyvinylidene fluoride membranes (ATTO, Tokyo, Japan). After blocking nonspecific reactions with Block Ace (Dainippon Pharmaceutical, Osaka, Japan), the membranes were incubated with rabbit polyclonal antibody against claudin-5 (1/250; Zymed, San Francisco, CA) or rabbit polyclonal antibody against β-actin (1/5000 dilution; Abcam, Cambridge, UK) for 1 hour at room temperature. After washing in PBS containing 0.1% Tween 20, the membranes were further reacted with horseradish peroxidase-conjugated anti-rabbit IgG (1:15,000 dilution; Amersham Biosciences Corp., Piscataway, NJ) for 30 minutes at room temperature. A chemiluminescence reagent, ECL Western blotting detection reagent (Amersham Biosciences Corp.), was used to make the labeled protein bands visible. For quantification, the density of each band was determined by the NIH Image 1.41 program (available at ftp from zippy.nimh.nih.gov/ or from http://rsb.info.nih.gov/nih-image; developed by Wayne Rasband, NIH, Bethesda, MD). The density of the claudin-5 band was standardized to that of β-actin, and the densities in normoxic and hypoxic bEND.3 cells or retinas were compared.
Immunofluorescence Microscopy
Cultured bEND.3 cells on fibronectin-coated dishes were fixed with 100% methanol for 5 minutes at room temperature and were incubated with 5% normal swine serum in PBS for 30 minutes at room temperature to block the nonspecific binding of antibodies. For in vivo studies, flat mounts of retinas were fixed with 100% methanol for 5 minutes at room temperature and were treated with PBS containing 5% normal swine serum and 0.5% Triton X-100 for 6 hours at room temperature to block nonspecific binding of antibodies and for the permeabilization of the tissues. Subsequently, the cells and the flat mounts were reacted with rabbit polyclonal antibody against claudin-5 (1/25; Zymed) at 4°C overnight. After washing with PBS, they were incubated with fluorescein isothiocyanate-conjugated swine polyclonal antibody against rabbit immunoglobulins (DakoCytomation Denmark A/S, Glostrup, Denmark) for 6 hours at room temperature. The stained cells and retinal flat mounts were then washed with PBS and mounted in fluorescent mounting medium (DakoCytomation) for observation under a confocal microscope (FluoView FV1000; Olympus, Tokyo, Japan).
Measurement of Transendothelial Electrical Resistance
bEND.3 cells were grown to confluence on fibronectin-coated cell inserts with 0.4-μm pore size, and the resistance of inserts was measured using the Millicell ERS Voltohmmeter (Millipore, Billerica, MA). The transendothelial electrical resistance (TEER) of the inserts was calculated by subtracting the resistance of blank inserts from that of the inserts with bEND.3 cells and multiplying the subtracted values by the area of the insert. The TEER was used as an index of the barrier property of the bEND.3 monolayer.
Transient Transfection of siRNA
The 21-oligonucleotide small interfering RNA (siRNA) with the sequence of claudin-5 (5′-AACATCGTTGTCCGCGAGTTC-3′) and control non-silencing (5′-AATTCTCCGAACGTGTCACGT-3′) oligonucleotides were chemically synthesized (QIAGEN, Germantown, MD). For annealing, 20 μmol/L siRNA in 30 mmol/L Hepes-KOH buffer, pH 7.4, containing 100 mmol/L CH3COOK, and 2 mmol/L (CH3COO)2Mg was incubated at 90°C for 1 minute and subsequently at 37°C for 1 hour. Then, using the Nucleofector kit (Amaxa, Gaithersburg, MD), 2 μg of siRNA was transfected into 1 × 106 bEND.3 cells suspended in 100 μl of Nucleofector solution V. Program T-20 was selected according to the manufacturer’s instructions to achieve a high transfection efficiency. The cells were then plated onto the fibronectin-coated culture dishes or cell inserts and further incubated in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum for Western blotting and the measurement of TEERs.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RT-PCR was performed to determine the mRNA level of claudin-5. The level of the mRNA of β-actin was used as the standard. Total RNA was extracted from the bEND.3 cells cultured under either normoxia or hypoxia for 24 hours using Isogen (Nippon Gene, Toyama, Japan), and total RNA (2 μg) was reverse-transcribed in a 15-μl reaction volume with a First-Strand cDNA Synthesis Kit (Pharmacia Biotech, Uppsala, Sweden) as described.33 One microliter of the reaction mixture was then subjected to PCR for amplification of each molecule. PCR was performed in 50 μl containing 800 nmol/L each primer, 250 nmol/L dNTPs, and 5 U of TaqDNA polymerase (Toyobo, Tokyo, Japan) with a thermal controller (MiniCycler; MJ Research, Inc., Watertown, MA). The number of PCR cycles was 30 for claudin-5 and 25 for β-actin. The thermal cycle was 1 minute at 94°C, 2 minutes at 60°C (claudin-5) or 57°C (β-actin), and 3 minutes at 72°C, followed by final extension for 3 minutes at 72°C. The nucleotide sequences of the PCR primers were 5′-GACTGCCTTCCTGGACCAC-3′ (forward) and 5′-TGACCGGGAAGCTGAACTC-3′ (reverse) for claudin-5; and 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′ (forward) and 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ (reverse) for β-actin. The expected sizes of the amplified cDNA fragments of claudin-5 and β-actin were 500 bp and 837 bp, respectively. The PCR products were electrophoresed on a 1.5% agarose gel and then stained with ethidium bromide.
Real-Time Quantitative Polymerase Chain Reaction (Real-Time PCR)
For quantitative analysis of the level of claudin-5 mRNA, TaqMan real-time PCR assay was performed using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Primers and TaqMan probes specific for claudin-5 and β-actin were purchased from Applied Biosystems (sequences not disclosed). The cycling conditions were 50°C for 2 minutes, initial denaturation at 95°C for 10 minutes, and then 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. β-Actin was used to normalize the amount of claudin-5 mRNA in each sample, and claudin-5 to β-actin mRNA ratio (claudin-5 mRNA/β-actin mRNA ratio) was compared between the bEND.3 cells under normoxia and hypoxia.
Detection of Tissue Hypoxia
The effect of hypoxic conditions on mouse retinas was determined by the Hypoxyprobe-1 Plus Kit (Chemicon International, Temecula, CA). One hour before sacrifice, pimonidazole hydrochloride (Hypoxyprobe-1; Chemicon International), which binds to the proteins in hypoxic cells, was injected into the peritoneal cavities of mice (60 mg/kg). Flat mounts of retinas were prepared and fixed with 100% methanol for 5 minutes at room temperature. After 6 hours of incubation with blocking buffer, retinal flat mounts were reacted with both the fluorescein isothiocyanate-conjugated mouse monoclonal antibody against Hypoxiprobe-1 (1/50 dilution) and the rat monoclonal antibody against CD31 (PECAM-1) (1/500 dilution; BD Biosciences, San Jose, CA) at 4°C overnight. The flat mounts were subsequently incubated with Alexa Fluor 546-conjugated goat antibodies against rat immunoglobulins (Molecular Probes, Eugene, OR) for 6 hours at room temperature. The retinas were stained with the anti-CD31 antibody to make the endothelial cells visible. The stained flat mounts were observed with a confocal microscope (FluoView FV1000; Olympus).
Tracer Experiments
To determine the permeability of the mouse retinal vessels, tracer experiments were performed as described.23 Under deep anesthesia with pentobarbital sodium, the mouse chest cavity was opened, and a 24-gauge cannula was inserted into the left ventricle. Each mouse was perfused with 500 μl/g body weight of PBS containing 100 μg/ml Hoechst stain H33258 (molecular mass, 534 d; Sigma) and 1 mg/ml tetramethylrhodamine-conjugated lysine-fixable dextran (molecular mass, 10 kd; Molecular Probes). The isolated retinas were flat mounted and observed with a confocal microscopy (FluoView FV1000; Olympus).
Statistical Analyses
All of the data are expressed as the means ± SD. The data were analyzed with Mann-Whitney tests, and differences were considered to be statistically significant at P < 0.05.
Results
Correlation between Expression of Claudin-5 and Induction of Barrier Function of Monolayer of bEND.3 Cells
bEND.3 cells that had reached confluence were cultured for an additional 11 days under normoxia, and the expression of claudin-5 was determined by Western blotting and immunocytochemistry. The barrier function of a monolayer of bEND.3 cells was also evaluated by measurements of the TEERs.
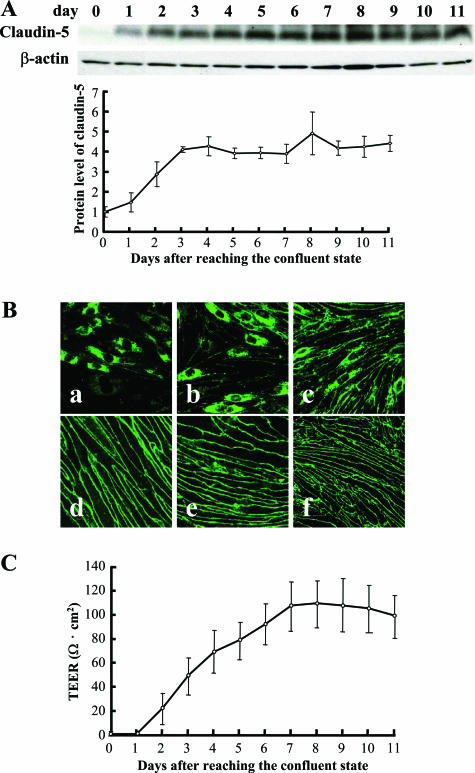
After the cultured bEND.3 cells reached confluence, the cellular protein level of claudin-5 increased and reached a steady-state level at day 3 (Figure 1A). In addition, the claudin-5 molecule relocated to the plasma membranes between adjacent bEND.3 cells at day 7 (Figure 1B). The detection of claudin-5 in the plasma membrane was strongly correlated with the increase in the TEER of the bEND.3 monolayer (Figure 1C).
Figure 1.
Claudin-5 expression and TEER in monolayer of bEND.3 cells. bEND.3 cells at the confluent state were cultured for an additional 11 days under normoxic condition, and the expression of claudin-5 (A, B) and barrier property of bEND.3 monolayer (C) was investigated. A: Western blotting with its quantitative analysis (bottom panel) showed that the cellular protein level of claudin-5 increased and reached a steady-state level around day 3, whereas the level of β-actin, a loading control, was almost unchanged. B: Immunocytochemistry showed that the predominant cytoplasmic location of claudin-5 immediately after reaching confluence (a) gradually changed to a relocation to the plasma membranes. Finally, claudin-5 was exclusively located to the plasma membranes 7 days after reaching confluence (d). a, 1 day; b, 3 days; c, 5 days; d, 7 days; e, 9 days; f, 11 days after reaching confluent state. C: The barrier property of bEND.3 monolayer was evaluated by measuring the TEER. A high correlation existed between the location of claudin-5 to the plasma membranes and the increase in the TEER of bEND.3 monolayer.
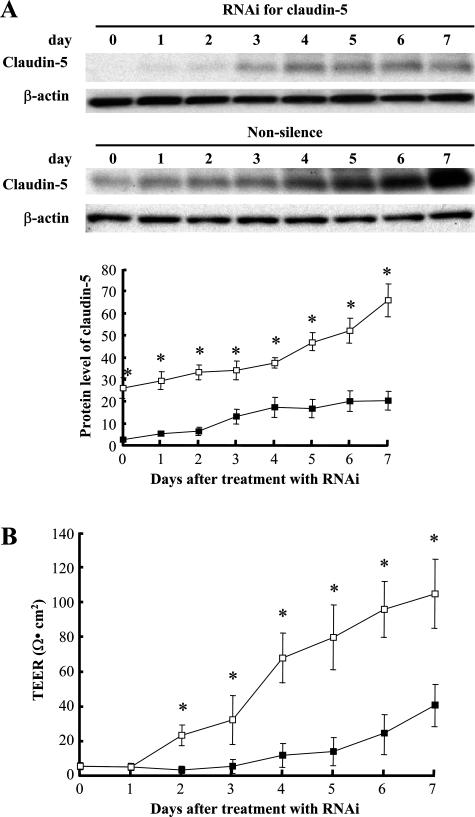
To evaluate the role of claudin-5 in the barrier function of bEND.3 monolayer in more detail, the RNAi technique was used to inhibit the expression of claudin-5. Monolayers of bEND.3 cells that had the expression of claudin-5 depressed failed to develop the increased TEER but did so with non-silencing oligonucleotides were used (Figure 2).
Figure 2.
Relationship between the TEER of the bEND.3 monolayer and the expression levels of claudin-5. A: Expression level of claudin-5 in bEND.3 cells was altered by the RNAi technique, and Western blotting with quantitative analysis (bottom panel) confirmed the depressed claudin-5 expression in cells that were treated with siRNA specific for claudin-5 compared with the cells treated with non-silence oligonucleotides. The level of β-actin was used as a control for protein loading. B: bEND.3 cells with depressed expression of claudin-5 (closed square) failed to develop the monolayer with the TEER equivalent to that of bEND.3 monolayer without the depression of claudin-5 (open square). *P < 0.01.
Hypoxia-Induced Changes in Claudin-5 Expression and Barrier Function of Monolayer of bEND.3 Cells
The expression of claudin-5 was analyzed and compared between the bEND.3 cells cultured under normoxic and hypoxic conditions. Confluent bEND.3 cells were cultured for an additional 7 days under normoxia to obtain the cells with claudin-5 exclusively located in the plasma membranes. The cells were then exposed for 24 hours to either normoxic or hypoxic conditions, and the expression of claudin-5 was evaluated by immunocytochemistry and Western blotting.
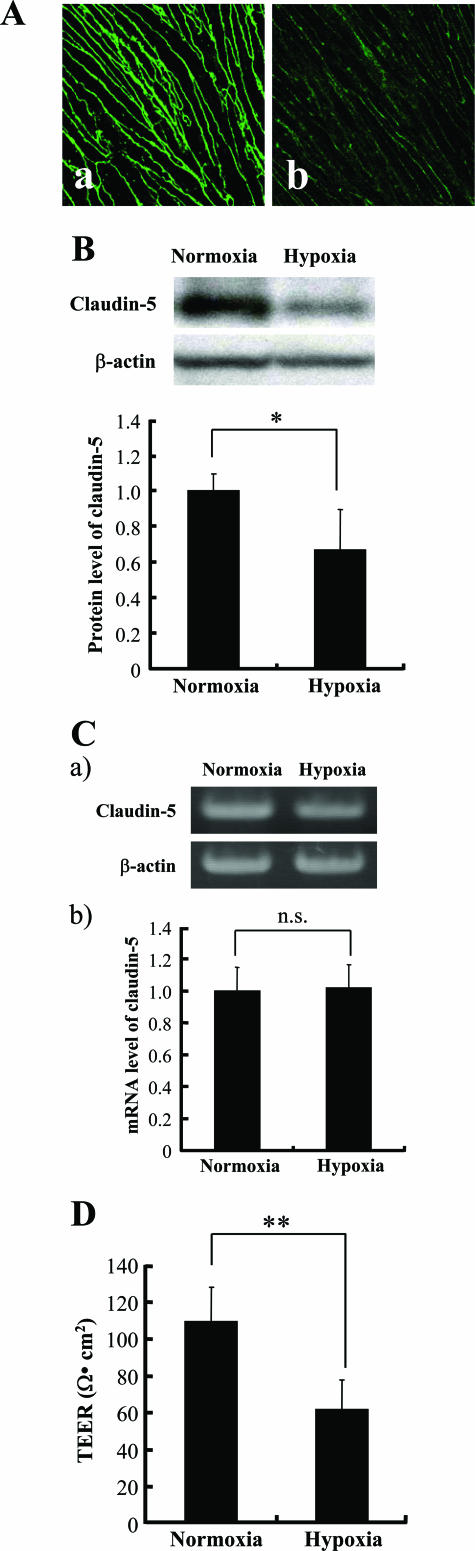
Our results showed that the amount of claudin-5 molecules in the plasma membranes was decreased under hypoxic conditions, which was accompanied by a decrease in their cellular protein levels (Figure 3, A and B). Interestingly, the changes in the expression level of the mRNA of claudin-5 mRNA were not significantly different between cells cultured under normoxia and hypoxia (Figure 3C). The hypoxic bEND.3 monolayer with diminished localization of caludin-5 to plasma membranes showed weak barrier properties with significantly lower TEER than that of the normoxic monolayer (Figure 3D). In addition, bEND.3 cells recovered from these hypoxic changes in claudin-5 expression and TEER when they were further incubated under normoxia for 48 hours (data not shown).
Figure 3.
Hypoxia-induced changes in the expression of claudin-5. bEND.3 cells with claudin-5 located in the plasma membranes were cultured under either normoxia or hypoxia for 24 hours. Immunocytochemistry (A) and Western blotting (B) with its quantitative analysis (bottom panel in B) revealed that the localization of claudin-5 to plasma membranes [A (a, normoxia; b, hypoxia] as well as the cellular protein level of claudin-5 decreased under hypoxic condition. C: The mRNA level of claudin-5 was determined by RT-PCR (a) and real-time quantitative PCR (b). The claudin-5 mRNA level was approximately the same under normoxic and hypoxic conditions. Protein and mRNA levels of β-actin were also quantified as the controls of those of claudin-5. D: TEERs of bEND.3 monolayers under normoxia and hypoxia were measured, and the barrier property of bEND.3 cells was disrupted by hypoxia. *P < 0.05, **P < 0.01.
In Vivo Expression of Claudin-5 in Retinal Blood Vessels under Normoxia and Hypoxia
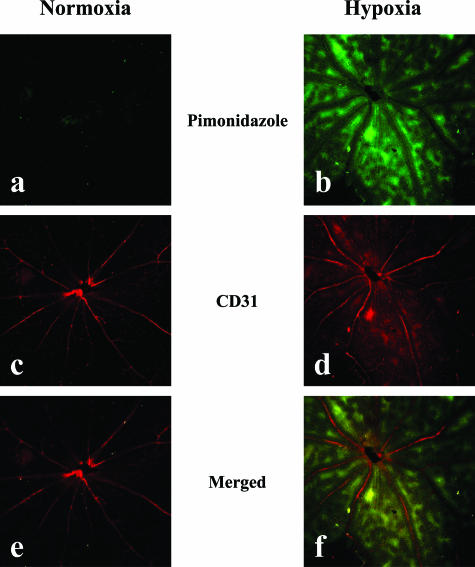
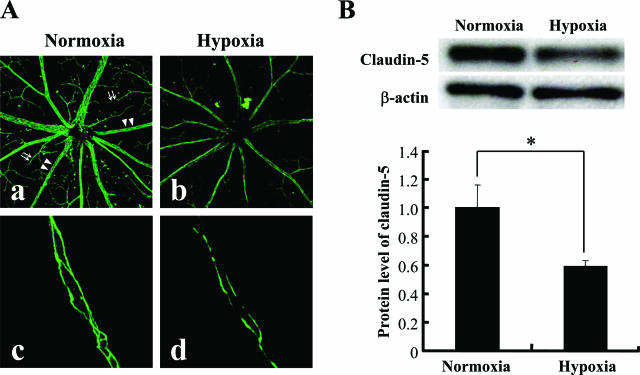
Mice were maintained in either atmospheric air or in decreased O2 concentration for 7 days, and then their retinas were processed for immunohistochemistry to investigate the in vivo expression of claudin-5 in the retinal blood vessels. Oxygenation of retinal tissues was evaluated by the intraperitoneal injection of pimonidazole hydrochloride, and it was confirmed that the retinal tissues from mice maintained under decreased O2 concentration were hypoxic as compared with those from mice in atmospheric air (Figure 4). Retinal blood vessels of mice in atmospheric air expressed claudin-5 with its distinct location at the interfaces of adjacent endothelial cells of both proximal and peripheral blood vessels. The expression of claudin-5 was reduced in the blood vessels of hypoxic retina from mice maintained in air with decreased O2 concentration (Figure 5A, a and b). A significant decrease (approximately 41%) in the claudin-5 expression of hypoxic retinas was confirmed by Western blotting (Figure 5B). Suppression of claudin-5 expression was predominantly observed in the peripheral small blood vessels, in contrast to its relatively preserved expression in the proximal blood vessels. In some of the capillaries, the signal for claudin-5 was hardly detected by immunohistochemistry (Figure 5A, c and d).
Figure 4.
Oxygenation of retinas from mice under normoxia or hypoxia. To evaluate the tissue hypoxia, pimonidazole hydrochloride (Hypoxyprobe-1) was injected into the peritoneal cavities of mice maintained under either normoxic or hypoxic conditions for 7 days, and pimonidazole hydrochloride incorporated into hypoxic cells was detected by immunostaining (a, b). Immunostaining for CD31 was performed to examine the retinal vasculature (c, d). Merged view (e, f) figured the hypoxic metabolism of the retinas from mice that had been maintained under hypoxia, especially in the areas distant from the blood vessels.
Figure 5.
In vivo effect of tissue hypoxia on the expression of claudin-5 in retinal blood vessels. Expression of claudin-5 in retinas from mice that had been maintained under normoxia or hypoxia for 7 days was investigated by immunofluorescence (A) and Western blotting (B). The level of β-actin was used as a loading control of Western blotting. A: Claudin-5 expression in the plasma membranes of retinal blood vessels is depressed in mice under hypoxia (b, d) in contrast to the distinct expression in the mice under normoxia (a, c). The decrease in claudin-5 expression by hypoxia was distinct in the peripheral blood vessels (arrows in a) compared with the reserved expression of claudin-5 in the proximal blood vessels (arrowheads in a) (c, d). B: Tissue protein level of claudin-5 significantly decreased in the hypoxic retina compared with the normoxic retina (top panel, gel of Western blotting; bottom panel, quantitative analysis of Western blotting for the relative decrease in claudin-5 expression in hypoxic retina). *P < 0.05.
Permeability of Retinal Blood Vessels with Reduced Expression of Claudin-5
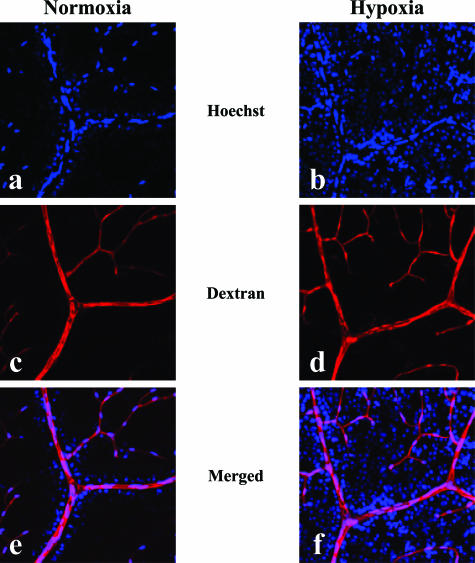
The permeability of the retinal blood vessels under normoxia and hypoxia was evaluated by tracer experiments using Hoechst stain H33258 (534 d) and tetramethylrhodamine-conjugated lysine-fixable dextran (10 kd). The injected dextran was detected in the vascular lumen with minimal leakage from blood vessels in both normoxic and hypoxic retinas (Figure 6). The extravasation of the Hoechst H33258 dye was manifested by the nuclear staining of surrounding retinal glial and neural cells, and the degree of staining was enhanced in the hypoxic retina as compared with that in normoxic retina (Figure 6).
Figure 6.
Tracer experiment to evaluate the permeability of retinal blood vessels in vivo under normoxia or hypoxia. Injected tracers, Hoechst stain (a, b) and dextran (c, d), in normoxic (a, c, e) and hypoxic (b, d, f) retinas were detected under confocal microscopy. Merged views (e, f) of the signals of Hoechst stain and dextran are presented. Extensive nuclear staining by the extravasated Hoechst stain was noted in a hypoxic retina (f), whereas the stained nuclei were localized only in the vicinity of vascular lumen in a normoxic retina (e). On the other hand, no significant leakage of the injected dextran was detected in both normoxic (e) and hypoxic (f) retinas.
Discussion
The hypoxia-induced breakdown of the barrier properties of neural blood vessels often accelerates the progression of diseases such as cerebral ischemic diseases and diabetic retinopathy.7,12 However, the molecular mechanisms involved with the barrier disruption by hypoxia have not been determined. Identification of the molecules responsible for the hypoxic disruption of endothelial barrier would yield the new therapeutic targets of the intractable diseases. We focused on claudin-5, which is a member of claudin family and is involved in the assembly of tight junctions between neural microvascular endothelial cells.22,23 Our results showed that the expression of claudin-5 is altered by hypoxia, which results in the disruption of the barrier properties of neural vascular endothelial cells.
When bEND.3 cells, which are derived from mouse neural vascular endothelial cells, were cultured in the confluent state under normoxic conditions, they formed a monolayer and acquired barrier properties. We found that bEND.3 cells expressed claudin-5, which was located predominantly in the cytoplasm just after they reached confluence. Under normoxic conditions, claudin-5 relocated to the plasma membranes at the interface of adjacent cells. This localization of claudin-5 was also well correlated with the development of the barrier properties of the bEND.3 monolayer, which was determined by measuring TEER. The location of claudin-5 in the plasma membrane and the increase in the TEER of confluent bEND.3 cells reached a steady-state level after the incubation for 7 days. Therefore, the maturation of confluent bEND.3 cells as neural microvascular endothelial cells was thought to be accomplished at day 7 after they reached confluence. The critical role of claudin-5 in the induction of barrier properties of endothelial cell monolayer was further confirmed by the specific inhibition of claudin-5 expression by RNAi, which blocked the increase in TEER of confluent bEND.3 monolayer even under normoxic conditions. These findings prompted us to analyze and compare the expression of claudin-5 in bEND.3 cells under normoxia and hypoxia to determine whether claudin-5 was a target molecule for the hypoxic disruption of barrier function of neural blood vessels. The data clearly demonstrated that hypoxia reduced the relocation of claudin-5 molecules to the plasma membranes, which was accompanied by a decrease in the protein level of claudin-5 in the cells. The functional significance of the hypoxia-induced changes in the expression of claudin-5 in endothelial cells was confirmed by the decrease in the TEER of the cellular monolayer.
To verify the effect of tissue hypoxia on the claudin-5 expression and subsequently on the barrier function of neural microvasculature in vivo, flat mounts of the retina were used because the entire vascular tree could be easily analyzed. Using the incorporation of pimonidazole hydrochloride into retinal cells as a measure, our data showed that the retinal tissues became hypoxic by placing mice in a chamber containing 7 to 9% O2 concentration. With this in vivo model, the degree of expression of claudin-5 in the endothelial plasma membranes was reduced in the hypoxic retina. This was accompanied by a decrease in the protein level of claudin-5 in the whole retina. These changes in the expression pattern of claudin-5 in the retinal endothelial cells in vivo were essentially identical to those observed in the cultured bEND.3 cells under hypoxic conditions. The hypoxic depression of claudin-5 expression was predominantly observed in the peripheral capillaries, whereas claudin-5 molecules in the endothelial cell membranes of proximal arteries were relatively well preserved, suggesting the critical role of claudin-5 in the barrier disruption in hypoxic retinas. The barrier function of retinal vasculature under normoxia and hypoxia was also assessed by using Hoechst stain H33258 (534 d) and dextran (10 kd) as tracers. The extravasation of the injected Hoechst stain was enhanced in the hypoxic retina compared with that in the normoxic retina. In contrast, dextran, a tracer molecule of larger size, remained inside the vessels in both normoxic and hypoxic retinas. Thus, the barrier function in hypoxic retinal blood vessels was selectively disrupted against small molecules, and this phenotype is identical to that of brain blood vessels in claudin-5-deficient mice.23 It was reported that there were molecules expressed differently between the immortalized retinal and brain capillary endothelial cell lines.34,35 Although it remains to be discussed whether our in vivo data of retinal blood vessels could be interpreted as the common findings of neural blood vessels, our in vitro and in vivo data indicate that claudin-5 is one of molecules responsible for the hypoxia-induced disruption of the barrier of neural blood vessels.
The expression and location of the tight junction molecules, such as claudins, occludin, ZO-1, and ZO-2, are finely controlled during embryonic development. The relocation of claudin-5 to the plasma membrane of neural endothelial cells was shown to be enhanced as the blood vessels mature.36 The appropriate expression of junctional molecules in epithelial and endothelial cells is crucial for the normal functioning of tissues, and disturbances of the expression of tight junction molecules, both congenital and acquired, cause various pathological conditions.21,37 Accumulating evidence has disclosed that the localization of claudins to cell junctions can be controlled transcriptionally, translationally, and posttranslationally, depending on the stimuli, and that the response to a stimulus is also different among the different members of the claudin family.21,38,39 It has been reported that exposure of brain-derived endothelial cells to hypoxic conditions resulted in the depressed localization of occludin, ZO-1, and ZO-2 to the plasma membrane, although their protein levels were little affected.8,9 A significant increase in the protein level of claudin-1 by hypoxic stimuli has also been reported.31 Through the screening of molecules whose mRNA levels in endothelial cells were controlled by the oxygen concentration, claudin-3 was found to be a hypoxia-sensitive molecule.25 In contrast, an in vivo study has shown that the level of claudin-3 in brain microvessels was unchanged following exposure to hypoxia and reoxygenation.40 Claudin-like protein of 24 kd (CLP24) was also identified as a cell junction protein which modulates the paracellular permeability in response to hypoxia, although it is associated with adherens junctions but not with tight junctions.41
In the present study, the disappearance of claudin-5 from the plasma membranes of hypoxic endothelial cells was accompanied by a decrease in the cellular protein level of claudin-5. In contrast, the level of claudin-5 mRNA was almost unchanged regardless of the oxygen concentration. Therefore, the hypoxia-induced changes in the expression of claudin-5 are attributable to the posttranscriptional regulation such as a reduced translation and the enhanced degradation of claudin-5 molecules. For the posttranscriptional regulation of total cellular protein level of tight junction molecules, a down-regulation of claudin-1 by a transcription factor, Snail,42 and a degradation of occludin in the proteasome pathway through the specific ubiquitination of occludin by Itch43 have been reported. It is possible that the ubiquitin-proteasome pathway is also involved in the hypoxic changes in claudin-5 expression through the degradation of claudin-5 molecules. The cleavage of claudin-5 molecules by proteases such as matrix metalloproteinases might be enhanced in hypoxic endothelial cells, because matrix metalloproteinases are suggested to play a role in the blood-brain barrier disruption.44 However, further studies are needed to clarify the mechanisms involved in the hypoxia-induced decrease in the cellular protein level of claudin-5.
Acknowledgments
We thank Hitoshi Abe, Department of Pathology, School of Medicine, Keio University, for skillful technical assistance.
Footnotes
Address reprint requests to Eiji Ikeda, Department of Pathology, School of Medicine, Keio University, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan, E-mail: eikeda@sc.itc.keio.ac.jp.
Supported by Grant-in-Aid for Scientific Research (C) 17590317 from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to E.I.) and a grant from the Takeda Science Foundation (to E.I.).
References
- Ikeda E, Flamme I, Risau W. Developing brain cells produce factors capable of inducing the HT7 antigen, a blood-brain barrier-specific molecule, in chick endothelial cells. Neurosci Lett. 1996;209:149–152. doi: 10.1016/0304-3940(96)12625-2. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Risau W, Wolburg H. Development of the blood-brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Saunders NR. Ontogeny of the blood-brain barrier. Exp Eye Res. 1977;25(Suppl):523–550. doi: 10.1016/s0014-4835(77)80046-8. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wiesnet M, Marti HH, Renz D, Schaper W. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J Cell Physiol. 2004;198:359–369. doi: 10.1002/jcp.10417. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm. 2006;113:477–485. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- Kniesel U, Risau W, Wolburg H. Development of blood-brain barrier tight junctions in the rat cortex. Brain Res Dev Brain Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, Duffner F, Grote EH, Risau W, Engelhardt B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol (Berl) 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- Scheurer SB, Rybak JN, Rosli C, Neri D, Elia G. Modulation of gene expression by hypoxia in human umbilical cord vein endothelial cells: a transcriptomic and proteomic study. Proteomics. 2004;4:1737–1760. doi: 10.1002/pmic.200300689. [DOI] [PubMed] [Google Scholar]
- Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- Ikeda E, Achen MG, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells. J Biol Chem. 1994;269:760–764. [PubMed] [Google Scholar]
- Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci USA. 1994;91:6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda E. Cellular response to tissue hypoxia and its involvement in disease progression. Pathol Int. 2005;55:603–610. doi: 10.1111/j.1440-1827.2005.01877.x. [DOI] [PubMed] [Google Scholar]
- Brown RC, Mark KS, Egleton RD, Huber JD, Burroughs AR, Davis TP. Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NFkappaB. J Cell Sci. 2003;116:693–700. doi: 10.1242/jcs.00264. [DOI] [PubMed] [Google Scholar]
- Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Ishida S, Inoue M, Obata K, Oguchi Y, Okada Y, Ikeda E. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2003;44:2163–2170. doi: 10.1167/iovs.02-0662. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Tomi M, Hata T, Nagai Y, Hori S, Mori S, Hosoya K, Terasaki T. Dominant expression of androgen receptors and their functional regulation of organic anion transporter 3 in rat brain capillary endothelial cells; comparison of gene expression between the blood-brain and -retinal barriers. J Cell Physiol. 2005;204:896–900. doi: 10.1002/jcp.20352. [DOI] [PubMed] [Google Scholar]
- Tomi M, Abukawa H, Nagai Y, Hata T, Takanaga H, Ohtsuki S, Terasaki T, Hosoya K. Retinal selectivity of gene expression in rat retinal versus brain capillary endothelial cell lines by differential display analysis. Mol Vis. 2004;10:537–543. [PubMed] [Google Scholar]
- Virgintino D, Errede M, Robertson D, Capobianco C, Girolamo F, Vimercati A, Bertossi M, Roncali L. Immunolocalization of tight junction proteins in the adult and developing human brain. Histochem Cell Biol. 2004;122:51–59. doi: 10.1007/s00418-004-0665-1. [DOI] [PubMed] [Google Scholar]
- Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, Chiba H. Tight junctions and human diseases. Med Electron Microsc. 2003;36:147–156. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res. 2003;290:275–288. doi: 10.1016/s0014-4827(03)00354-9. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247–1257. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- Witt KA, Mark KS, Hom S, Davis TP. Effects of hypoxia-reoxygenation on rat blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2003;285:H2820–H2831. doi: 10.1152/ajpheart.00589.2003. [DOI] [PubMed] [Google Scholar]
- Kearsey J, Petit S, De Oliveira C, Schweighoffer F. A novel four transmembrane spanning protein, CLP24: a hypoxically regulated cell junction protein. Eur J Biochem. 2004;271:2584–2592. doi: 10.1111/j.1432-1033.2004.04186.x. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- Traweger A, Fang D, Liu YC, Stelzhammer W, Krizbai IA, Fresser F, Bauer HC, Bauer H. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J Biol Chem. 2002;277:10201–10208. doi: 10.1074/jbc.M111384200. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600375. [Epub ahead of Print] [DOI] [PubMed] [Google Scholar]