Abstract
Connexin 43 (Cx43) is thought to be present largely in the plasma membrane and its function solely to provide low resistance electrical connection between myocytes. A recent report suggested the presence of Cx43 in the mitochondria as well. We confirmed the presence of Cx43 in the mitochondria isolated from adult rat ventricles with the Cx43 immunoreactivity fractionating to the outer mitochondrial membrane. Mitochondrial Cx43 is mostly phosphorylated only detected by a phospho-specific antibody. Using a Ca++-sensitive electrode and Western blot, we showed that the gap junction inhibitors 18-β-glycyrrhetinic acid (β-GA), oleamide, and heptanol all induced concomitant release of Ca++ and cytochrome C in isolated mitochondria whereas the inactive analog 18-β-glycyrrhizic acid failed to do so. In low density neonatal myocyte culture with no appreciable cell-cell contacts, β-GA induced apoptosis as assessed by TUNEL staining. Our results suggest a novel role of Cx43 as a regulator of mitochondrial physiology and myocyte apoptosis.
Keywords: mitochondria, connexins, apoptosis, β-glycyrrhetinic acid, gap junction inhibitors, cytochrome C, TUNEL, cardiac myocytes
Introduction
Cellular apoptosis plays a critical role in cardiovascular diseases [1, 2]. While many regulators of this programmed cell death pathway have been identified the list is clearly incomplete. Connexin (Cx) 43, the major gap junction forming protein in the adult cardiac ventricles, plays a pivotal role in mediating tissue injury and post-ischemic cardiac dysfunction, however, the mechanism by which Cx43 modulates cellular injury is not known.
Most of the function ascribed to Cx43 in cardiac pathophysiology is within the context of its role in forming the gap junction [3]. However, recent literature reports atypical Cx43 functions in roles outside of the traditional gap junction [4, 5]. We recently discovered that Cx43, independent of its ability to form functional gap junctions, regulates susceptibility of cells to several cell injury paradigms suggesting a novel function of this protein in modulating cell death [6]. Cx43 is abundantly expressed in the adult cardiac ventricles, yet little study exists addressing the role of cardiac Cx43 in modulating myocyte death. Myocyte apoptosis is now recognized to mediate cell death in a variety of acute and chronic heart diseases and understanding how Cx regulates apoptosis may lead to a new novel approach to preventing myocyte death.
A recent report suggested that Cx43 is present in the mitochondria and may play a role in mediating the cardioprotective effect of ischemic preconditioning [7]. In the present study, we confirmed the presence of Cx43 immunoreactivity in the outer membrane of the mitochondria isolated from adult cardiac ventricles and provide evidence that mitochondrial Cx43 as a novel regulator of mitochondrial function where inhibition results in the release of cytochrome C and myocyte apoptosis.
Methods
Isolation of rat ventricular mitochondria and fractionation
All procedures were conducted in accordance with institutional animal care regulations. Minced rat ventricular tissue chunks were suspended in a mannitol-sucrose (M/S) buffer (in mM): 255 mannitol, 10 sucrose, 0.5 EGTA, 1 glutathione, 10 HEPES, pH 7.4, and cells lysed by 10 strokes of a Dounce homogenizer. The first low speed centrifugation at 2000g x 10 min separated the unlysed cells and the nuclei. The supernatant from the first spin (S1) was re-spun at 6000g x 10 min and the pellet (P2) was collected as the fraction enriched in mitochondria. For the mitochondria functional assay, the P2 pellet was resuspended in the M/S buffer without EGTA and oxygen applied by blow-by until used. For protein isolation, the P2 pellet was resuspended in RIPA buffer (1% Nonidet P40, 10mM Tris pH 7.6, 50mM NaCl, 30mM NaPPi, 50mM NaF, 1% Triton X100, and 0.1% sodium dodecyl-sulfate) and sonicated (50% power for 2 second x 10 pulses, Cole Palmer Model 130 Ultrasonic Processor). All procedures were done at 4C° or on ice.
Subcellular fractionation of the organelles by idioxanol gradient (19-27%) centrifugation was carried out according to manufacturer’s instructions (Axis-Shield, Oslow, Norway). For the sub-mitochondrial fractionation, the P2 pellet suspended in M/S buffer supplemented with 1 mM DTT and 0.2 mM PMSF was sonicated, as noted above, followed by 3 freeze-thaw cycles (modified from Beutner et. al., [8]). The mitochondrial fragments were loaded on a sucrose gradient (from 60% to 30%) in 10 mM HEPES, pH7.4, supplemented with a protease inhibitor cocktail (Complete, Roche Applied Science, Indianapolis, IN, USA), and centrifuged at 100,000g for 3 hours at 4C° (Beckman Optima TLX centrifuge with a TL55 swinging bucket rotor).
Western blotting
Proteins subjected to SDS-PAGE and transferred to a nitrocellulose membrane was blocked in 3% milk-TBST and probed with the following primary antibodies: ANT (1:500, Santa Cruz Biotech, Santa Cruz, CA, USA; sc-11433); Calreticulin (1:500, Affinity Bioreagents, Golden, CO, USA; #PA1-903); Cx43 (1:500, Chemicon International, Temecula, CA, USA; MAB3067), GAPDH (1:100,000, Advance Immunochemical, Long Beach, CA, USA; #RGM2); N-cadherin (1:1000, Zymed Lab, South San Francisco, CA, USA; #18-0224), VDAC (1:1000, Oncogene, Boston, MA, USA; #PC548), all in 3% milk-PBST, and reacted with the horseradish peroxidase-conjugated secondary antibody (1:2000) in 1% milk-TBST. After reaction with the Western Lightning chemiluminescence reagent (NEN Life Science Products, Boston, MA, USA), the images were captured on the EpiChemi Darkroom System (UVP Inc, Upland, CA). The commercially available anti-Cx43 antibody raised against a peptide-epitope (pan-Cx43) used in this study is well-characterized specifically recognizing both the non-phosphorylated and phosphorylated Cx43 protein with little non-specific reactivity [9]. The Cx-1B1 anti-Cx43 antibody (#13-8300, Zymed Lab,) only recognizes Cx43 when the serine at residue 368 is unphosphorylated (P0-Cx43) [10]. Dephosphorylation was accomplished by incubating mitochondrial lysate with calf intestinal alkaline phosphatase (10 U) at 37C° for 20 hours and separated by a 12% SDS-PAGE to optimize separation of multiple bands in the 40-50kD range.
Immunohistochemistry
Cultured cardiomyocytes were fixed with 4% paraformaldehyde in 0.1M PB for 15 minutes. After permeablization with 0.2% Tween-20 in phosphate buffered saline (PBST), and blocking (10% normal goat serum in PBST) both for 15 minutes at room temperature, the samples were incubated with the following antibodies: Cx43 antibody (1:200), VDAC (1:200), and α-actinin (1:500, Sigma-Aldrich, St.Louis, MO, USA) all in PBST with 2% normal goat serum for 2 hours at room temperature. After washing, the cells were reacted with the appropriate secondary antibody conjugated to Alexa 487 or 594 (1:500, Molecular Probes, Portland, OR, USA), and visualized under a Zeiss LSM 510 NLO confocal microscope (Oberkochen, Germany) and psuedo-colored in Photoshop.
Measurement of mitochondrial calcium uptake
Mitochondrial suspension in MS buffer without EGTA was placed in a stirred container and the free-[Ca++] within the solution adjusted to approximately 10 μM and thereafter continuously monitored by a Ca++-sensitive electrode (MI-600, Microelectrodes, Inc, Bedford, NH, USA) connected to an Accumet 25 pH meter (Fisher Scientific, Pittsburg, PA, USA). The voltage output was calibrated using solutions of known free-[Ca++] (WP Instruments, Sarasota, FL, USA) and demonstrated a superior log-linearity over a wide range of [Ca++].
Myocyte culture and apoptosis cell count assay
Rat neonatal ventricular myocytes were isolated from day 2-4 neonatal pups by enzymatic digestion following a procedure recommended by the manufacturer (Worthington, Lakewood, NJ, USA). After pre-panning the remaining myocyte-enriched suspension was plated at a low density (50,000 cells/35mm well). The growth of background cells was inhibited by addition of cytosine arabinoside (1 μM) 24 hours after plating and thereafter, the culture was maintained in DMEM supplemented with 10% newborn calf serum, 10% equine serum, and 1% penicillin/streptomycin.
For the apoptotic cell count assay, myocytes were treated with β-GA (0-100 μM) or its inactive analogue (GZ, 100 μM) for 24h in the serum containing medium. The presence of the serum reduced the total number of apoptotic cells making the visualization and counting of apoptotic cells easier. Fixed (4% PFA) myocytes were subjected to the terminal deoxyribonucleotide transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay as per manufacturer’s instructions (Promega, Madison, WI, USA). The cells counts were taken from no less than 12 random fields, where the total number of myocytes counted was typically 1000 cells. Data (mean ± SEM) is expressed as % TUNEL-positive.
Chemicals
All chemicals were purchased from Sigma-Aldrich except for carbonlycyanide-4-(trifuloromethoxy)-phenylhydrazone (FCCP) (Biomol, Plymouth Meeting, PA, USA) and MitoTracker (Invitrogen, Carlsbad, CA, USA).
Results
Anti-Cx43 immunoreactivity in the cardiac mitochondria:
Immunoreactivity to anti-Cx43 antibody was investigated in cultured rat neonatal ventricular myocytes. The culture prepared by a standard method consisted of myocytes and non-myocyte support cells; however, the myocytes were easily identified because of their characteristic stellate morphology and the immunoreactivity to anti-α-actinin antibody. Immunostaining with anti-Cx43 antibody (Figure 1A, red) demonstrated numerous punctate widely distributed immunoreactive spots in addition to the expected linear streaks at the cell borders. The punctate immunoreactive spots resembled the distribution of mitochondria in these cells which was confirmed by MitoTracker staining (Figure 1A, green) and by anti-VDAC antibody immunoreactivity (data not shown). However, inspection of the images indicated that the two markers only partially overlapped.
Figure 1.
Anti-connexin-43 antibody immunoreactivity in cardiac mitochondria. A. The anti-Cx43-immunoreactivity (red) of some low density cultured rat neonatal ventricular myocytes was peri-nuclear and punctate visible throughout the cell reminiscent of the MitoTracker green staining (green). Calibration bar = 20 μm. B. Crude homogenate of adult rat heart ventricles was separated by two rounds of centrifugation as described in the Methods. P1=first pellet, S1=first solute, P2=second pellet and equal protein amount (30 μg) was loaded in each lane. Br=total brain lysate served as a control and the immunoreactivity to the following proteins served as markers for cellular compartments: VDAC (mitochondrial marker), GAPDH (cytosolic marker), N-Cad (plasma membrane marker). C. The P2 pellet from the centrifugation was further fractionated by iodixanol gradient and equal volume from the 13 collected fractions was loaded and proteins analyzed by immunoblotting. The far right lane of the crude homogenate served as a positive control for the respective antibody immunoreactivity. D. The mitochondria present in the P2 pellet was separated into the sub-mitochondrial fractions as described in Methods. Equal protein amount (15 μg) from the respective fractions was loaded and proteins analyzed by immunoblotting. ANT (inner mitochondrial membrane marker), VDAC (outer mitochondrial membrane marker), IMM (inner mitochondrial membrane), CS (contact site), OMM (outer mitochondrial membrane), Br (brain). Representative of 3 Western blots from two separate mitochondrial membrane fractionation preparations. E. Mitochondrial lysate was subjected to dephosphorylation by alkaline phosphatase and probed with antibody recognizing all Cx43 regardless of the phosphorylation status of the protein (Pan-Cx Ab) or with non-phosphorylated-species specific antibody (P0-Cx Ab). Arrows point to the putative phosphorylated Cx43 species.
Localization of Cx43 in low density cultured neonatal rat ventricular myocytes maybe abnormal, therefore, we attempted to demonstrate the presence of anti-Cx43 immunoreactivity in the adult ventricular mitochondria by biochemical organelle and protein fractionation methods. A crude fractionation of cellular organelles was accomplished by a simple 2-step centrifugation and the isolated fractions probed by immunoblotting (Figure 1B). The following immunoreactive signals were used as markers: anti-VDAC (mitochondrial marker), anti-GAPDH (cytosolic marker), and N-cadherin (plasma membrane marker). The P2 pellet isolated by this method mostly separated the cytosolic and the plasma membrane compartments away as demonstrated by the minimal GAPDH and N-cadherin immunoreactivity in this fraction. The P1 fraction with the plasma membrane exhibited robust anti-Cx43 immunoreactivity as expected. However, the P2 fraction also demonstrated robust immunoreactivity suggesting the presence of Cx43 in a non-plasma membrane and non-cytosolic compartment of the ventricular cells.
Further fractionation of the organelles was attempted using the P2 pellet as the starting material. Fractions collected after an iodixanol-gradient centrifugation demonstrated anti-Cx43, anti-VDAC, and anti-calreticulin (endoplasmic reticulum marker) immunoreactivity in overlapping fractions (Figure 1C). However, the anti-Cx43 immunoreactive fractions tended towards the less dense fraction compared to the overlapping fractions immunoreactive to anti-VDAC and anti-calreticulin. As expected, we saw no immunoreactivity to N-cadherin or GAPDH in the fractions since the starting P2 pellet was mostly devoid of these signals.
Next we followed the anti-Cx43 immunoreactivity in sub-fractions of the mitochondria again starting with the P2 pellet but by separating the sub-mitochondrial protein compartments by sucrose gradient centrifugation after lysis of the mitochondria. Immunoblotting with anti-VDAC as a marker for the outer mitochondrial membrane and with anti-ANT as a marker for the inner mitochondrial membrane confirmed the proper isolation of the sub-mitochondrial compartments (Figure 1D). The contact-site (CS) showed immunoreactivity to both the inner- and outer-mitochondrial membrane markers as expected. The anti-Cx43 immunoreactivity in the total mitochondria appeared as multiple distinct bands (arrows) with apparent masses slightly greater than the expected 43kD. Immunoreactivity in the fractionated samples was present in the CS and a stronger signal in the outer mitochondrial membrane but only of the larger apparent mass. Since Cx43 is a phosphoprotein that exhibits a well described phosphorylation-induced mobility shift, we examined the phosphorylation-status of the mitochondrial Cx43 by probing with a P0-specific anti-Cx43 antibody. Mitochondrial lysate probed with the pan-Cx43 antibody detected multiple bands (Figure 1E, arrows) that collapsed to a single band on alkaline phosphatase treatment. The anti-P0-Cx43 antibody detected a band only when dephosphorylated indicating that majority of the Cx43 immunoreactive species present in the mitochondria is phosphorylated. These observations confirmed the report that Cx43 was present in the mitochondria itself and not just in close proximity [9] and extended this observation by suggesting that the mitochondrial Cx43 is phosphorylated.
Gap junction inhibitors cause a loss of mitochondrial integrity
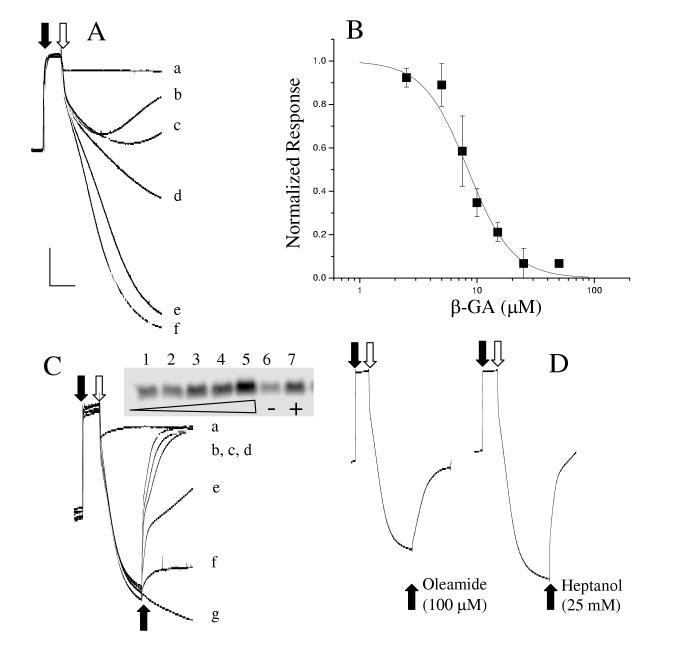
We next investigated the possible function mediated by mitochondrial Cx43 by examining the effects of several well-characterized gap junction inhibitors on the mitochondrial calcium uptake. Live mitochondria isolated in the P2 fraction were added to a buffer and the free-[Ca++] increased to approximately 10 μM initiating the active uptake of Ca++ by the ruthenium red-sensitive mitochondrial Ca++-uniporter. Control mitochondria exhibited a robust Ca++ uptake demonstrated by a large decrease in the buffer free-[Ca++]. Pre-treatment of the mitochondria with the gap junction blocker 18β-glycyrrhrtinic acid (β-GA) either inhibited the ruthenium red-sensitive Ca++ uptake or resulted in a premature release of Ca++ from the mitochondria (Figure 2A) resulting in a less dramatic decrease in the free- [Ca++] in the buffer. This effect was β-GA concentration-dependent (Figure 2B). To distinguish between these two possibilities, mitochondria were first loaded with Ca++ and β-GA added subsequently. Addition of β-GA to mitochondria pre-loaded with Ca++ caused a release of Ca++ and a concomitant release of cytochrome C (Figure 2C) consistent with the idea that inhibition of the mitochondrial gap junction induced loss of mitochondrial integrity. Identical experiments with β-glyzhrrhtic acid (β-GZ), a derivative of β-GA but devoid of the gap junction inhibitory effect, demonstrated no release of mitochondrial Ca++ or cytochrome C. Two other well-characterized and chemically distinct inhibitors of the gap junction, heptanol and oleamide [11, 12], induced an analogous release of Ca++ from the mitochondria (Figure 2D). Taken together, the data suggested that inhibition of mitochondrial gap junction leads to the loss of mitochondrial integrity and release of cytochrome C. However, a non-gap junction related action common to the three drugs acting on the mitochondria can not be ruled out.
Figure 2.
Gap junction inhibitors release Ca++ and cytochrome C from isolated cardiac mitochondria. A. CaCl2 was added to the buffer (closed arrow) to adjust the [free-Ca++] to approximately 10 μM. Mitochondria preincubated with β-GA (in μM: 25 (b), 10 (c), 5 (d), 2.5 (e), 0 (f), or 100 ruthenium red (a)) added to the buffer (open arrow) initiated a time-dependent Ca++ uptake resulting in a decrease in the buffer Ca++concentration. B. Concentration-dependence of inhibition of Ca++-uptake from n=3 pre-incubation experiments. IC50 = 8.4 ± 1.2 μM, Hill slope = 2.29 ± 0.60. C. Mitochondrial Ca++ - uptake was initiated as in A. Concentration-dependent release of Ca++ by the acute addition (bottom solid arrow) of β-GA (in μM: 25 (b), 20 (c), 15 (d), 10 (e), 5 (f)) or ruthenium red 100 μM (a) in Ca++-loaded mitochondria. GZ (50 μM) (g) with no known effect on gap junction function did not induce Ca++-release. Inset: A Western blot for cytochrome C released by the mitochondria (1: none, 2: 5μM, 3: 10μM, 4: 25μM, 5: 50 μM all β-GA, 6: 50 μM GZ, 7: 100μM FCCP. Equal protein loading was confirmed by probing for GAPDH (not shown). D. Two other well known gap junction inhibitors of distinct chemical structures release Ca++ from the mitochondria in the same protocol as described for C.
β glycyrrhrtinic acid induces myocyte apoptosis
If β-GA induced cytochrome C release at the organelle level, the consequence should be an induction of apoptosis at the cellular level. Indeed, neonatal rat ventricular myocytes demonstrated a β-GA concentration-dependent increase in the number of apoptotic cells assessed by TUNEL staining (Figure 3). At 100 μM β-GA, approximately 7% of the myocytes were TUNEL-positive, while in cultures treated with β-GZ essentially none were destined to apoptosis.
Figure 3.

β-glyccerhetic acid induces apoptosis in cultured neonatal rat ventricular myocytes. A. Cells were treated with β-GA (0-100 μM) (panels i-iv) or its inactive analogue (GZ, 100 μM, panel v) for 24h in DMEM containing serum. Cells were then fixed in 4% PFA and myocytes we identified by immunocytochemical staining for α-actinin (red). Apoptotic cells were determined by TUNEL labeling (green) and nuclei by Hoechst stain (blue). The images are composite overlay of all three channels, B. Summary histogram of % TUNEL positive myocytes for different β-GA concentrations (n=3 independent experiments). Cells counts were taken from no less than 12 random fields, where the total number of myocytes counted was typically 1000 cells. Bars represent mean ± SEM.
Discussion
Cx43 protein in the mitochondria
Early immunohistochemical studies documented anti-Cx43 antibody immunoreactivity on the plasma membrane in close apposition to the mitochondria leading to the suggestion that mitochondrial sequestration of Ca++ may play a role in Cx43 gating. [13]. Our Western blot results confirmed the presence of Cx43 immunoreactivity in the sub-mitochondrial membrane fraction enriched in the outer mitochondrial membrane. A recent report concluded that the mitochondrial Cx43 protein localized to the inner mitochondrial membrane because of its co-immunoprecipitation in a macromolecular complex with the inner-mitochondrial membrane protein ANT, outer-mitochondrial protein Tom20 translocase, and Hsp90 heat shock protein [14]. The reason for our discrepancy with this report is not clear but further co-immunoprecipitation studies with inner mitochondrial membrane translocases and reversing the antibodies for immunoprecipitation and immunodetection should provide further insight on the exact localization of Cx43 within the mitochondria.
More significantly, our functional assay demonstrated that gap junction inhibitors blocked a ruthenium-red sensitive calcium uptake which is a hall mark of the mitochondrial calcium uniporter [15] in contradistinction to the SERCA-ATPase responsible for the calcium uptake into the endoplasmic reticulum [16]. Taken together, our data suggested the presence of Cx43 in the mitochondria itself and not just in close proximity, exists largely as phospho-Cx43, and plays a role in the regulation of mitochondrial function.
The MitoProt II software (www.mips.biochem.mpg,de/cgi-bin/proj/medgen/mitofilter) predicts mitochondrial localization of a protein based on an analysis of the signal peptide [17]. A query for connexins returns a probability of mitochondrial-targeting greater than 0.20 for connexin 25, 31.1, 31, 32, and 45 suggesting the possibility of these connexin isoforms also being present in the mitochondria. However, the mitochondrial-targeting probability is only 0.03 for the full length Cx43 protein. It is likely that Cx43, particularly when phosphorylated, may be imported into the mitochondria by specific translocases independent of the N-terminal signal sequence like many other mitochondrial proteins [18] possibly in association with a chaperone protein [14].
Functional consequence of inhibition of mitochondrial gap junction
Evidence for a functional role of mitochondrial Cx43 comes from studies investigating the effects of gap junction active-drugs on mitochondrial function. In rat liver mitochondria, β-GA was shown to induce MPT and release cytochrome C [19]. However, in their discussion, no association was made between the well known gap junction inhibiting-property of β-GA and the observed effect on mitochondrial physiology. We expanded upon this observation and presented in our present work that the parent glycyrrhizic acid (GZ) incapable of gap junction inhibition does not induce mitochondrial Ca++release. Furthermore, two other chemically distinct gap junction inhibitors, heptanol and oleamide, induced mitochondrial calcium release. However, no drugs exhibit absolute specificity and further studies using highly specific molecular reagents will be necessary to confirm our observations being mindful of the likelihood that in organs expressing multiple isoforms of Cx, simultaneous use of multiple selective inhibitors may be necessary to alter the mitochondrial function. In fact, the observation that β-GA induces MPT in the liver where the predominant Cx isoform is Cx32 and the lack of gross developmental abnormality in Cx43 null-mutant mice allowing survival to term [20] support the idea that multiple Cx isoforms must regulate mitochondrial physiology.
The hexameric Cx43 protein complex connexon, both as a gap junction and as a hemichannel, forms large-conductance ion channel with chemical gating similar to the Bcl-2 channels. In addition, Cx43 channels are voltage gated perhaps allowing sensing of the mitochondrial membrane potential in addition to the chemical environment. Given the well-documented role of mitochondrial ion channels such as Bcl-2 in modulating apoptosis, it is highly probable that the ion channels formed by the Cx43 protein may also play a role in modulating apoptosis, however, no evidence exists to indicate that the mitochondrial Cx43 forms an ion channel. Proving this hypothesis will require further experiments with a targeted mitochondrial over-expression of dominant-negative Cx43 mutants (e.g. L160M-mutant) incapable of forming ion channels [6] and examining the apoptotic phenotype in such cells.
Acknowledgements
We thank Dr. Shey Shing Sheu (University of Rochester) for introducing the area of mitochondrial physiology to the senior author and Mahmud Uzzaman for participating in early experiments. This work was partially supported by NIH RO1 GM071485 (JY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–71. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gonzalez A, Fortuno MA, Querejeta R, Ravassa S, Lopez B, Lopez N, Diez J. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc Res. 2003;59:549–62. doi: 10.1016/s0008-6363(03)00498-x. [DOI] [PubMed] [Google Scholar]
- [3].Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T. Gap junction alterations in human cardiac disease. Cardiovasc Res. 2004;62:368–77. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- [4].Kalvelyte A, Imbrasaite A, Bukauskiene A, Verselis VK, Bukauskas FF. Connexins and apoptotic transformation. Biochem Pharmacol. 2003;66:1661–72. doi: 10.1016/s0006-2952(03)00540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Plotkin LI, Bellido T. Bisphosphonate-induced, hemi-channel-mediated anti-apoptosis through the Src/ERK pathway: A gap junction-independent action of connexin 43. Cell Comm Adhes. 2001;8:377–82. doi: 10.3109/15419060109080757. [DOI] [PubMed] [Google Scholar]
- [6].Lin JH, Yang J, Liu S, Takano T, Wang X, Gao Q, Willecke K, Nedergaard M. Connexin mediates gap junction-independent resistance to cellular injury. J Neurosci. 2003;23:430–41. doi: 10.1523/JNEUROSCI.23-02-00430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana R, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–44. doi: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- [8].Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–8. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- [9].Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptide react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–05. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nagy JI, Li WEI, Roy C, Doble BW, Gilchrist JS, Kardami E, Hertzberg EL. Selective monoclonal antibody recognition and cellular localization of an unphosphorylated form of connexin 43. Exp Cell Res. 1997;236:127–36. doi: 10.1006/excr.1997.3716. [DOI] [PubMed] [Google Scholar]
- [11].Rozental R, Srinivas M, Spray DC. How to close a gap junction channel. Efficacies and potencies of uncoupling agents. Methods in Molecular Biology. 2001;154:447–76. doi: 10.1385/1-59259-043-8:447. [DOI] [PubMed] [Google Scholar]
- [12].Boger DL, Patterson JE, Guan X, Cravatt BF, Lerner RA, Gilula NB. Chemical requirements for inhibition of gap junction communication by the biologically active lipid oleamide. Proc Natl Acad Sci. 1998;95:4810–5. doi: 10.1073/pnas.95.9.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Forbes MS, Sperelakis N. Association between mitochondria and gap junctions in mammalian myocardial cells. Tissue Cell. 1982;14:25–37. doi: 10.1016/0040-8166(82)90004-0. [DOI] [PubMed] [Google Scholar]
- [14].Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, Konietzka I, Miro E, Totzeck A, Heusch G, Schulz R, Garcia-Dorado D. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circulation Res. 2006;99:1–9. doi: 10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- [15].Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–4. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- [16].Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–49. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- [17].Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241:779–86. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- [18].Rehling P, Brandner K, Pfanner N. Mitochondrial import and the twin-pore translocase. Nat Rev Mol Cell Biol. 2004;5:519–530. doi: 10.1038/nrm1426. [DOI] [PubMed] [Google Scholar]
- [19].Salvi M, Fiore C, Armanini D, Toninello A. Glycyrrhetinic acid-induced permeability transition in rat liver mitochondria. Biochem Pharmacol. 2003;66:2375–9. doi: 10.1016/j.bcp.2003.08.023. [DOI] [PubMed] [Google Scholar]
- [20].Reaume AG, de Soursa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Caridac malformation in neonatal mice lacking connexin 43. Science. 1995;267:1831–4. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]