Abstract
This review summarizes comprehensively the most important and representative molecular genetics studies of gene identification for osteoporosis published up to the end of December 2004. It is intended to constitute a sequential update of our previously published review covering the available data up to the end of 2002. Evidence from candidate gene association studies and genome-wide linkage studies in humans, as well as quantitative trait locus mapping animal models are reviewed separately. Studies of transgenic and knockout mice models relevant to osteoporosis are summarized. An important extension of this update is incorporation of functional genomic studies (including DNA microarrays and proteomics) on osteogenesis and osteoporosis, in light of the rapid advances and the promising prospects of the field. Comments are made on the most notable findings and representative studies for their potential influence and implications on our present understanding of genetics of osteoporosis. The format adopted by this review should be ideal for accommodating future new advances and studies.
Keywords: osteoporosis, molecular genetics, association, linkage, functional genomics
INTRODUCTION
We recently reviewed the important findings made in the field of genetics of osteoporosis, which summarized data up to the end of 2002.(1) Since its publication, the review has received considerable attention and is of help for osteoporosis research. This constitutes strong encouragement for us to review the literature in extenso periodically to provide an up-to-date and objective evaluation on the endeavors to identify genetic factors underlying risk of osteoporosis. In this article, we aim to provide an update to our previous review(1) to summarize the molecular genetics studies published within 2003–2004. The data presented in this review were collected by systematic search through PubMed. The key words of “bone,” “BMD,” “osteoporosis,” “polymorphisms,” “linkage,” “QTL,” “osteogenesis,” “microarray,” “proteomics,” etc. were used in the search.
As with our first review,(1) this synthesis includes sections dealing with association studies and linkage studies in human populations, quantitative trait loci (QTL) mapping in animal models, and transgenic and knockout murine models relevant to osteoporosis. A notable extension is inclusion of functional genomic studies, owing to the rapid progress in the field and its promising prospects in understanding biochemistry of proteins, processes, and pathways relevant to osteogenesis and osteoporosis. The results of important studies are entered in the tables for comparison and for ease of reference. Table 1 (cited online) summarizes the major results from ~170 reported candidate gene association studies. Because transgenic/knockout animal models continue to reveal novel pathways and candidate genes for bone mass regulation, we list in Table 2 (cited online) the studies that have appeared over the past few years. Genome-wide linkage scans in humans and QTL mapping in model organisms are elaborated on and summarized individually in Tables 3 and 4, respectively. Table 5 (cited online) incorporates DNA microarray studies on osteogenesis, osteoporosis, and other bone-related diseases. For findings and studies reported before 2002, the readers may refer to our first review.(1)
Several articles are available that address the strategies(2–4) and status of genetic study of osteoporosis.(5–14) We recently overviewed the powerful and promising methodologies in the study of complex bone disorders at the whole genome level.(15) In addition, we systematically addressed the confounding factors that cause nonreplication in gene mapping of osteoporosis and proposed tentative remedies.(16) Given that, a detailed discussion on the above aspects will not be attempted here. Because of space constraints, we are unable to reference all original studies in this review. With apologies to the omitted authors, the reader is referred to other reviews for detailed descriptions.
CANDIDATE GENE ASSOCIATION STUDIES
During the past two years, ~170 association studies were published (Table 1, cited online). The candidate genes involve classical genes (e.g., VDR, ER-α, and COL1A1) that have been extensively studied, as well as novel genes (e.g., LRP5 and SOST) recently discovered to be important in bone and mineral metabolism. The newly studied genes include CYP17 (17-α-hydroxylase),(17) CYP1B1 (cytochrome P450),(18) DBP (vitamin D–binding protein),(19) GH1 (growth hormone 1),(20) GnRH (gonadotropin-releasing hormone 1),(21) IGF-II (insulin-like growth factor II),(22) LEPR (leptin receptor),(23) LRP5 (low-density lipoprotein receptor–related protein 5),(24) BMP2 (bone morphogenetic protein 2),(25) CCR2 (chemokine),(26) CLCN7 (chloride channel 7),( 2 7 ) COMT (catechol-O-methyltransferase),(28) CTSK (cathepsin K),(29) DRD4 (dopamine receptor D4),(30) I-TRAF (TRAF family member-associated NF-κB activator),(31) LCT (lactase),(32) MIF (macrophage migration inhibitory factor),(33) MMP-1 (matrix metalloproteinase 1),(34) MMP-9 (matrix metalloproteinase 9),(35) NCOA3 (nuclear receptor co-activator 3),(36) NPY (neuropeptide Y),(37) OSCAR (osteoclast-associated receptor),(38) PLOD1 (procollagen-lysine, 2-oxoglutarate 5-dioxygenase),(39) PON1 (paraoxonase 1),(40) RIL (LIM domain protein RIL),(41) SERT (serotonin transporter),(42) SOST (sclerostin),(43) and TCIRG1 (T cell immune regulator 1).(44)
In the following, we highlight some studies performed in samples of at least 1000 subjects. This is because statistical power is among the foremost factors for robust and replicable results, and generally, at least 1000 subjects are needed to detect modest genetic effects (e.g., a QTL explaining 5% of phenotypic variation) in a population-based association study.(16) Some meta-analyses were addressed, because meta-analyses, by combining results across studies, are helpful to solve the problems of underpowered studies, revealing unexpected sources of heterogeneity, and resolving discrepancies in genetic studies.(45) Other association studies are cited in Table 1 (cited online). For the classical candidate genes, their potential physiological effects on bone metabolism and pathophysiological implications to osteoporosis have been elaborated elsewhere(1,14); for the novel genes, their potential functions will be briefly outlined.
Classical candidate genes
VDR
Association between the VDR gene and osteoporosis-related traits has been extensively studied.(1) The frequently studied markers include BsmI, ApaI, TaqI, and FokI, which currently have unknown functional effects.(1) A novel polymorphism at the Cdx-2 (an intestine-specific homeodomain-containing transcription factor) binding site of the promoter region was identified to be able to activate VDR gene transcription and was associated with BMD variation in a Japanese population.(46)
Yamada et al.(25) studied the 2C-T (i.e., FokI) polymorphism with BMD variation in ~1100 Japanese women and ~1100 Japanese men. BMD did not differ among the 2C-T genotypes for all women, whereas men bearing the CT genotype had lower BMD than those with the CC genotype at the distal radius, femoral neck, and Ward’s triangle (p ≤ 0.05).(25)
Morita et al.(47) randomly selected 50 women from each of the 5-year age-stratified groups (15–79 years) in three Japanese municipalities, that is, 650 subjects for each area and 1950 in total. After excluding subjects who had medical or menstrual histories affecting BMD, 1434 women were analyzed for ApaI, TaqI, and FokI polymorphisms. TaqI was significantly associated with the distal one-third radius BMD in premenopausal women (p = 0.019). Analyzing three major combined genotypes (aaTT, AaTT, AaTt) of ApaI and TaqI revealed even stronger association for the distal one-third radius BMD in premenopausal women (p = 0.009). However, analyses on major haplotypes (AT or aT) failed to detect any significant association. In addition, they tested the relationship between the three polymorphisms and BMD change over 3 years in 976 subjects.(47) The annual percent changes in lumbar spine BMD of the TaqI tt subjects were different from other genotypes in women who were premenopausal at the follow-up survey and in the women who were postmenopausal at the baseline survey. However, the effect of the tt genotype on BMD change was opposite in the two groups. The authors concluded that none of the individual polymorphisms were consistently associated with baseline BMD or BMD change, and hence, the effect of the VDR genotype on BMD is negligible in Japanese women.
Fang et al.(48) examined Cdx-2 with BMD and risk of fracture in a cohort of 2848 Dutch ≥55 years of age. They did not find significant association for BMD, but they detected borderline association with vertebral fracture (p = 0.04) and any type of fractures (p = 0.06) with subjects carrying Cdx-2 A allele having reduced relative risk (RR) of fracture by 20%.(48) The protective effect of the A allele was similar in women and men.
Thakkinstian et al. reported two meta-analyses.(49,50) One study focused on the relationship between VDR BsmI and BMD/osteoporosis at the femoral neck or spine in adult women.(49) In that study, BsmI was associated with spine BMD in postmenopausal (p = 0.028) but not pre-menopausal women. The association was modest and followed a recessive model, with the BB genotype having lower BMD than Bb/bb genotype. The magnitude of the decrease in spinal BMD by BB genotype was 2.4%, which translated into a population attributable risk of spine fracture of 1.98%.(49) They also studied the association between BsmI and mean percent BMD change over time. Analyses revealed a significant effect (p = 0.017), with BB and Bb genotypes having greater bone loss per year than the bb genotype. The other meta-analysis was conducted with data on BsmI, ApaI, and TaqI, as well as on haplotypes defined by them.(50) Although none of the individual polymorphisms was associated with osteoporosis on their own, they found significant association between spinal osteoporosis and haplotypes BAT (p < 0.001) and BaT (p = 0.031), with ORs of ~4. For spine BMD, the only association was found for BsmI in postmenopausal Asian women (p < 0.001), suggesting that the genetic effect of VDR BsmI could be population-, menstrual status–, and site-dependent. This was in agreement with their previous meta-analysis.
Based on the available data, Morrison wrote a commentary on association between VDR and BMD.(51) By using the data provided in one meta-analysis mentioned above,(49) the author indicated that the effect size of the BsmI polymorphism on BMD was ~0.11–0.13 SD in post-menopausal women. To detect such modest effects, large sample sizes are required. For instance, based on the estimated allele frequency, 3046 whites or 4700 Japanese are required to have 80% power to detect the effect of 0.13 SD at p = 0.01.
Recently, two studies assessed the linkage disequilibrium (LD) pattern by examining multiple single nucleotide polymorphisms (SNPs) across the gene.(52,53) Nejentsev et al.(53) resequenced the gene and genotyped 55 common SNPs (minor allele frequency > 10%) in four European populations and one African population. LD patterns were identical (with three block-like regions) in all four European populations, but with two additional LD-breaking spots in an African population. In another study, Fang et al.(52) sequenced 22 kb of the gene (including the promoter, all exons, and the 3′ UTR) and identified 62 SNPs. LD analyses on common SNPs revealed four to eight haplotype blocks, which were less fragmented in whites and Asians than in Africans. They subsequently tested 15 tagging SNPs with BMD and fracture risk in 6535 elderly whites. Two haplotypes (containing the Cdx-2 SNP and 3′UTR, respectively) were associated with increased risk for vertebral fractures or overall osteoporotic fractures. The combined risk alleles showed 46% increased risk for vertebral fracture (p = 0.03) and 34% increased risk for osteoporotic fracture (p = 0.01).(52) This whole gene analysis suggested that VDR polymorphisms in the promoter region and 3′UTR may interactively affect fracture risk. In another study, the same group found tagging SNPs encompassing the 3′UTR of the VDR gene interacted with a SNP in the lactase-phlorizin hydrolase (LPH) gene, a variant correlated to lactose intolerance, to influence height, bone geometry, and BMD.(54)
The LD and haplotype-based association studies have several potential advantages.(55) First, haplotypes reduce the dimension of association tests and may gain statistical power because of increased information by combining multiple SNPs. Second, genetic variation in populations is intrinsically organized into haplotypes. Third, the folding kinetics, stability, or other physical properties of proteins may depend on interactions between pairs or higher-order combinations of amino acid sites. If these interactions are important, haplotypes are of direct biological relevance. For the observed associations with haplotype(s), it is necessary to assess their functions.
To date, >150 studies have been reported on associations between VDR gene polymorphisms and bone-related traits. Based on the available data, a tentative conclusion is that VDR gene polymorphisms, individually or interactively, may have effects on a number of biological endpoints, including BMD variation and bone fractures. Moreover, VDR may interact with nongenetic factors (i.e., calcium intake and estrogens) to modulate BMD. Although the effects of the VDR gene could be modest, they can still confer significant influence in the general population with their high frequencies. A similar example is the PPAR-γ Pro12Ala polymorphism, which is associated with a modest (1.25-fold) increase in diabetes risk.(56) Because the risk allele of Pro12Ala is so common in human populations, its modest effect translated into a large population attributable risk—influencing as much as 25% of type 2 diabetes in the general populations.(56)
The interpretation of VDR polymorphisms is currently hindered by the fact that most studies have investigated only a limited polymorphisms (e.g., BsmI, ApaI, TaqI, and FokI), which have unknown effects. The whole gene analyses that explore all potential sequence variations within/ around the gene will be helpful to identify the functional variants. It is also imperative to clarify the molecular mechanisms underlying the observed associations by conducting functional studies using well-defined cell types and/ or animal models.
ER-α
Three ER-α common polymorphisms, PvuII (T→C) and XbaI (A→G) in intron 1 and the TA repeat in the promoter region, have been extensively studied. The TA repeat polymorphism was speculated to affect bone mass by changing mRNA production or stability, whereas the functional relevance of PvuII and XbaI on bone mass remains to be elucidated. A possible mechanism is that the two polymorphisms are in LD with nearby functional variant(s), resulting in positive associations.
Yamada et al.(57) examined the relationship between PvuII and XbaI and BMD in ~2230 Japanese subjects 40–79 years of age. In women ≥60 years old, XbaI alone or in combination with PvuII showed significant association with femoral neck BMD. Zhao et al.(58) examined eight SNPs spanning the ER-α gene in 405 white nuclear families (1873 subjects). Marginal evidence was observed for femoral neck BMD with rs932477 (p = 0.015) and rs2228480 (p = 0.010). The most common seven-SNP haplotype (TCGCGGG) was associated with higher lumbar spine BMD (p = 0.015). Another study in 401 Chinese nuclear families (1260 subjects) reported a within-family association (p = 0.054) between spine BMD and the XbaI genotypes.(59)
Van Meurs et al.(60) studied the association of PvuII, XbaI, and TA repeat polymorphisms with BMD, vertebral bone area, and fractures in 2042 elderly Dutch whites. In women, subjects homozygous for haplotype px and a low number of TA repeats have significantly lower lumbar spine BMD (p = 0.003 and 0.008, respectively) and decreased vertebral bone area (p = 0.016) than homozygous noncarriers. They also found an increased vertebral fracture risk with an allele dose effect of OR = 2.2 for haplotype px and OR = 2.0 for a low number of TA repeats.
A meta-analysis was reported for PvuII, XbaI, and promoter TA repeats with BMD and fractures in 18,917 individuals from eight European research centers.(61) None of the three polymorphisms or haplotypes thereof had statistically significant association with BMD. However, there was a highly significant protection conferred by the XbaI XX genotype against fracture risk. In women with the XX genotype, the OR was 0.81 (p = 0.002) for any fractures and 0.65 (p = 0.003) for vertebral fractures. The observed effects on fractures were independent of BMD.
As with the VDR gene, the individual contribution of ER-α polymorphisms to osteoporosis remains to be universally confirmed. Future endeavors will be to elucidate their functional molecular relevance and their interaction with the environment in the causation of osteoporosis. A promising application of these polymorphisms is their pharmacogenomic implications, with the possibility of providing better guidance for therapeutic regimens, such as estrogen replacement therapy and selective ER modulators.
COL1A1
Thirteen association studies were reported over the past 2 years for the COL1A1 gene, many focusing on the Sp1 polymorphism, a G→T substitution at the first base of a consensus site in the first intron for the transcriptional factor. It is notable that association of COL1A1 Sp1 with osteoporotic fractures is among those mostly replicated.(62)
In 1044 elderly Swedish women, Gerdhem et al.(63) found association between COL1A1 Sp1 and femoral neck BMD (p = 0.027) and prevalent wrist fracture (p = 0.024). Women carrying at least one copy of the s allele had lower femoral neck BMD and higher prevalence of wrist fracture. The ORs for prevalent wrist fracture were 2.73 and 1.4 for ss and Ss genotypes, respectively, suggesting the pronounced effect of the s allele on increasing risk of wrist fractures. In 401 Chinese nuclear families, Zhang et al.(64) found that a -1997 G/T polymorphism in the COL1A1 upstream regulatory region was associated with hip BMD, explaining 1.6% of the total hip BMD variation. By testing three COL1A1 SNPs in 405 white nuclear families, Long et al.(65) reported that subjects bearing the T allele of SNP2 had, on average, 3.05% smaller wrist size than noncarriers.
Mann and Ralston(66) performed a meta-analysis for COL1A1 Sp1 and BMD/osteoporotic fractures, which involved 7849 subjects from 26 published studies. The Ss genotype group had significantly lower lumbar spine BMD than the SS group (p = 0.00005), but the difference between the SS and ss groups in spine BMD did not reach a significant level (p = 0.13). The femoral neck BMD were lower in the Ss group (p < 0.00001) and in ss group (p = 0.001) versus the SS group. They also found increased OR for any fracture in Ss subjects (OR = 1.26, p = 0.002) and an even greater increase in ss subjects (OR = 1.78, p = 0.003). Subgroup analysis showed that increased risk was largely attributable to vertebral fracture where the OR was 1.37 (p = 0.0004) and 2.48 (p < 0.00001) for Ss and ss subjects, respectively. Their results suggested that the COL1A1 Sp1 alleles contribute to a modest reduction in BMD and a significant increase in risk of osteoporotic fractures, particularly vertebral fractures.
The functional importance of Sp1 has been studied.(67) The s allele had greater affinity for the Sp1 protein compared with the S allele. In the Ss heterozygotes, the RNA transcripts derived from the s allele were three times more abundant than those from the S allele, suggesting allele-specific transcription or a different splicing process for the two alleles. The yield strength of bone derived from Ss individuals was reduced compared with bone derived from SS subjects.(67) With the availability of SNP information of the human genome, together with the ongoing HapMap project,(68) further studies are necessary to identify more causative variants in the COL1A1 gene using the LD mapping approach.
Other classical candidate genes
MTHFR (5,10-@methylenetetrahydrofolate reductase) affects the methylation of homocysteine to methionine, and high serum homocysteine concentrations have adverse effects on bone.(69,70) A MTHFR polymorphism, C677T, causes an alanine to valine substitution and gives rise to a thermolabile variant of MTHFR with reduced activity.(71) MTHFR C677T was associated with elevated levels of circulating homocysteine(72) and lumbar spine BMD.(73) In a study(74) using 1748 healthy postmenopausal Danish women, the TT genotype of the MTHFR C677T polymorphism was associated with lower BMD at the femoral neck, total hip, and spine (p < 0.05), with the effect sizes ranging from 0.1 to 0.3 SD. In consistency with BMD, the fracture incidence was increased >2-fold in subjects with the TT genotype. Although this variant did not affect the response to hormone replacement therapy (HRT), the association of the TT genotype with lower BMD was maintained at the total hip after 5 years of HRT.
Because the effect of C677T on circulating homocysteine levels was dependent on plasma folate concentration,(75) one study in 1632 whites evaluated whether the folate status may modify the association between C677T and BMD or quantitative ultrasonography (QUS) parameters.(76) The results showed that adjusted mean QUS parameters and BMD measurements did not significantly differ between C677T groups. However, suggestive interactions between folate status and the C677T group (CC + CT versus TT) were found for hip BMD (p ≤ 0.05) and for one of the QUS parameters, broadband ultrasound attenuation (BUA; p = 0.11). In subjects with low folate concentration (<4 ng/ml), the TT group had lower mean BUA (p = 0.06) and Ward’s area BMD (p = 0.08) compared with the CC + CT group; whereas in subjects with high folate levels (≥4 ng/ml), the TT group had significantly higher hip BMD (p ≤ 0.05). Because dietary B vitamins (folate, vitamin B12, vitamin B6, and riboflavin) can also influence circulating homocysteine levels, a study further exploited the association between C677T and BMD and bone loss, in relation to B vitamin intake in a cohort of perimenopausal and early postmenopausal Scottish women.(77) Although no association was observed for BMD, bone loss, or biochemical markers of bone turnover, there was a significant interaction between C677T and riboflavin intake in relation to BMD. BMD was lower for the TT group at low intakes of riboflavin compared with the other genotype, but at high intakes, BMD was higher in the TT group. The results suggested that the association between C677T and bone phenotypes might depend on B vitamin levels, especially on folate and riboflavin status. The mechanism underlying this modification remains unclear.
ApoE is a lipid-transporting glycoprotein that exists in three isoforms, encoded by three alleles, ApoE2, ApoE3, and ApoE4. A large population-based association study tested the effects of ApoE on BMD, bone loss, and osteoporotic fractures in 5857 Dutch subjects. No significant association was found with baseline BMD at the lumbar spine and femoral neck or with bone loss in a mean follow-up of 2.0 years. During a mean follow-up of 6.6 years, 708 non-vertebral fractures and 149 incident vertebral fractures occurred, but no significant association was observed between ApoE polymorphisms and incidence of these fractures. Another study(78) tested whether ApoE polymorphisms interact with vitamin K transport or with dietary fat to influence BMD and bone loss in 2481 early postmenopausal Scottish women. The only association was found between BMD change at femoral neck (p = 0.023) in alcohol drinkers (median alcohol intake = 7.3 g/day). A study(79) in 387 white nuclear families reported that APOE haplotypes defined by four SNPs may influence BMD in white males but not females.
IGF-I, by stimulating the proliferation of chondrocytes in the growth plate, plays an essential role in longitudinal bone growth. It is also involved in the formation of trabecular bone and is essential for coupling matrix biosynthesis to sustain mineralization. Plasma IGF-I levels were associated with BMD and osteoporotic fractures.(80,81) One study examined the role of a CA repeat polymorphism in the promoter region with hip BMD in a group of elderly Dutch women and men.(82) A total of 5648 and 4134 individuals underwent examination at baseline and a 2-year follow-up, respectively. Lower baseline BMD and higher BMD loss were associated with the absence of the 192-bp allele in women (p = 0.03), but not in men. This polymorphism only explained a minor portion of the variance in BMD (0.2%) and BMD change (0.1%) among females. The same group conducted another study evaluating association of the CA repeat polymorphism with incidence of nonvertebral fractures in 2799 men and 4212 women.(83) They also estimated the effects of this polymorphism on several hip bone geometry parameters, including neck width, cortical thickness, buckling ratio, and section modulus.(83) Women who were noncarriers or heterozygotes of the 192-bp allele had increased risk (1.5 and 1.2, respectively) of osteoporotic fractures compared with homozygotes for this allele (p = 0.0007). This effect was not observed in men. For hip geometry parameters, noncarrier males had narrower femoral neck and lower section modulus (p < 0.05) than homozygote men, whereas noncarrier females had thinner cortices and higher buckling ratios (p < 0.05) but no significant differences in femoral neck width and section modulus. The observed genotype-dependent differences in fracture risk cannot be fully explained by the genotype-dependent effects on hip bone geometry.
IL-6 is a pleiotropic cytokine that promotes the differentiation of osteoclast precursor cells into mature osteoclasts.(84) Yamada et al.(25) examined the -634C-G polymorphism of the IL-6 gene alone and in combination with the 298C-T polymorphism of the osteocalcin gene in ~2200 Japanese subjects (~1100 men and ~1100 women). Both polymorphisms were associated with BMD of total body and lumbar spine in postmenopausal women (p < 0.05). Analyses with combined genotypes suggested the two polymorphisms exert an additive effect on BMD in postmenopausal women. Another study in 3376 white women ⩾65 years of age reported association for distal and proximal radius BMD with the IL-6 G174C polymorphism (p = 0.016 and 0.049, respectively).(85) The risk of wrist fractures decreased by 17% per copy of C allele significantly (p = 0.043). Moreover, compared with women having the GG phenotype, women having the CC genotype also had slower rates of bone loss in the total hip and femoral neck in ~3.5 years of follow-up and 33% lower risk of wrist fractures over an average of 10.8 years.
Several other classical candidate genes have also been studied. In ~1300 Australian elderly women, a study examined A986S polymorphism of the CASR gene and T986C polymorphism of the TGF-β gene in relation to BMD, calcaneal QUS, and prevalent and incident fracture.(86,87) The TGF-β C allele was associated with lower BMD of the total hip, femoral neck, and trochanter, as well as QUS parameters. This allele was also associated with an increase in osteoporosis (T score ≤ −2.5 SD) and fracture risks.(87) In contrast, the CASR A986S polymorphism was not associated with any parameters.(86) Shearman et al.(88) assessed four intronic SNPs and one microsatellite marker near the ER-β gene in 723 men and 795 women and observed association with hip BMD in both men and women. OPG (a decoy receptor of RANKL) neutralizes biological activity of RANKL that functions as a potent agonistic ligand through binding to its receptor, RANK, on osteoclast lineage cells.(89) Two polymorphisms in the promoter of the OPG gene were examined in ~1100 Japanese women and ~1100 Japanese men.(90) The association was only found in women for BMD at various skeletal sites.(90) In a longitudinal study including 1792 women with or without HRT, two polymorphisms of the CYP19 gene were associated with the magnitude of BMD increase in response to HRT.(91) Albagha et al.(92) assessed the relationship between variants of the TNFRSF1B (i.e., TNFR2) gene and BMD in 1240 perimenopausal women from the United Kingdom. Notably, the TNFRSF1B gene is located on chromosome 1p36, a region linked to BMD in several genome-wide linkage studies.(1) Four polymorphisms, one in exon (T676G) and three in 3′ UTR (G593A, T598G, and T620C), were selected based on their potential functions.(92) No association was found between T676G and spine or hip BMD, but a haplotype (A593-T598-C620) was associated with femoral neck BMD, even after correction for multiple testing (p < 0.0009).(92) The TNF-α G308A polymorphism was evaluated in relation to BMD, bone structural geometry, and fracture risk in 4402 elderly women.(93) The G308A polymorphism was associated with femoral neck subperiosteal width and endocortical diameter, as well as bone bending strength. This polymorphism was also correlated with hip fracture risks during a 12.1-year follow-up, which was independent of its effects on BMD or bone strength.
Novel candidate genes
LRP5 was recently found to be a key regulator of osteoblast proliferation and bone formation. LRP5 mutations resulted in high bone mass phenotypes or osteoporosis-pseudoglioma syndrome.(94,95) In 219 Korean men 20–34 years of age, Koh et al.(96) examined six missense variants (Q89R, A400V, V677M, R1036Q, A1330V, A1525V), which were first identified in whites, with BMD at the lumbar spine and hip. Four of them (A400V, V677M, R1036Q, A1525V) were not polymorphic in Korean men, and the only association was found between Q89R and BMD at Ward’s triangle (p = 0.043). Ferrari et al.(97) examined the LD patterns using 13 previously reported SNPs in whites and selected 5 informative SNPs (a minor-allele frequency ≥ 5% and selected only one SNP for each pair of markers in nearly complete LD; r2 ≥ 0.9) for further association analysis. They ruled out potential population stratification using the program STRUCTURE.(98) Significant associations were found between 2047G-A (i.e., V677M) and lumbar spine BMD (p = 0.041), BMC (p = 0.0032), bone area (p = 0.0014), and stature (p = 0.0062). The observed associations with lumbar spine BMC and bone area were driven mainly by men. Haplotype analyses suggested that additional LRP5 genetic variants might also influence lumbar spine bone mass and area. They also found association between LRP5 haplotypes with 1-year bone mass and area changes in prepubertal males but not females. Taken together, the results suggested that LRP5 polymorphisms may influence lumbar spine bone mass and size in men, probably by affecting vertebral bone growth during childhood.(97) However, their claimed replication to a previous linkage reported by Koller et al.(99) might be problematic, because the linkage peak was detected in postmenopausal women,(99) but the association was found in males only.(97) Contrast to the male-specific effects observed by Ferrari et al.,(97) a study in 1301 elderly Australian women showed that the LPR5 gene was associated with hip bone mass and osteoporotic fractures.(100)
SOST is a disease-causing gene for sclerostenosis, a sclerosing bone dysplasia characterized by hyperostosis and overgrowth of normal bone tissue.(101) In a case-control study of 619 Scottish women with either high or low BMD (i.e., those in the upper or lower 16th percentile), Balemans et al.(43) identified 26 polymorphisms and selected 5 for association analyses. The distribution of genotypes and alleles for each tested SNP did not differ in the low or the high BMD group. Another study analyzed eight SOST polymorphisms in 1939 elderly Dutch men and women (55–80 years of age).(102) A 3-bp insertion in the promoter region (SRP3) was associated with decreased BMD in women at the femoral neck (p = 0.05) and lumbar spine (p = 0.01), with evidence of an allele–dose effect in the oldest age group (p = 0.006). The corresponding effect size between extreme genotypes was 0.2 SD. The polymorphism SRP9 was associated with femoral neck BMD (p = 0.007) and lumbar spine BMD (p = 0.02) in men, with the effect size between extreme genotypes of 0.2 SD. However, haplotype analyses failed to confirm the association observed in single SNP analyses. They further studied interactions between SOST polymorphisms and the VDR 3′UTR polymorphisms, as well as the COL1A1 Sp1 polymorphism.(102) An additive effect was observed between the SOST and COL1A1 Sp1 polymorphisms. The SOST–COL1A1 additive effect increased with age and reached 0.5 SD difference in lumbar spine BMD in the oldest age group (p = 0.02).
Giraudeau et al.(29) screened the CTSK gene in the promoters, exons, intron A, and all intron–exon boundaries in 130 random whites. Based on the LD pattern, they examined five CTSK polymorphisms and haplotypes thereof in relation to spine and hip BMD in ~3000 perimenopausal Scottish women. None of the polymorphisms or haplotypes exhibited significant association.
A number of other newly recognized candidate genes are listed in Table 1 (cited online) including CCR2, COMT, CYP 17, DRD4, IRAK1, MMP-1, MMP-9, PON1, and PON2s. Most of them were evaluated in only one study and await further study.
Summary
Association results in the bone field are currently inconsistent/inconclusive. A few articles have addressed this from perspectives of study design, analytical methodology, and others.(16,103–107) In the following, we re-emphasize important issues that undermine the validity of association studies.
First, a large portion of studies are underpowered (Fig. 1), which may yield unreliable/unrepeatable results.(16,108) Meta-analysis is a powerful tool for pooling previous research when individual studies have insufficient power.(49,66) However, meta-analysis is not always a panacea, because it is prone to biases resulting from different ascertainment and diagnostic criteria and population stratification in different studies.(16) Moreover, there exists publication bias favoring positive results, which further exacerbates the situation. We suggest a mechanism to publish negative results in a brief report section of specialty journals, and storage and dissemination of all association data, perhaps in a widely accepted web site, such as the recently developed Genetic Association Database (http://geneticassociationdb.nih.gov). Second, limited number of polymorphisms was evaluated in the target gene(s). Given the complexity of LD and haplotype patterns across the human genome and among different populations,(109–112) this may not provide a comprehensive and accurate evaluation of the gene(s) of interest.(16,113) Third, given multiple markers and/or phenotypes tested in a single study, it is too liberal to set the significance threshold at p ≤ 0.05, which, however, has inappropriately been used in many studies. Traditional Bonferroni correction is overconservative, because of nonindependence of the tests. Other methods accounting for multiple testing of correlated hypotheses have been developed.(114–116) Fourth, population stratification, a confounding factor for association analyses,(117) was not appropriately assessed and controlled. Although the actual impact of population stratification on association studies has been a matter of some debate,(118–120) modest amounts of population stratification was indeed detected in case-control and case-cohort studies(121,122) and even in a relatively homogenous genetic isolate.(123) A family-based association approach (e.g., transmission disequilibrium test [TDT]) could be effective to deal with the problem.(124,125) For population-based studies, methods to control population stratification have been developed (e.g., Genomic Control(126) and Structured Association.(127)), which, bearing in mind some assumptions, merit application and further development. Finally, other factors such as heterogeneity, epistasis, and gene–environment interaction have posed special challenges to gene discovery. Endeavor to maximize sample homogeneity by recruiting subjects from the same ethnic group and rigorous controlling possible environmental or confounding factors may increase the chance of success. Studies have suggested that there exists sex-specific genetic contributions to BMD.(128,129) The genes regulating peak bone mass might differ from those regulating bone loss.(130) It is imperative for association studies to be well designed, statistically powerful, and appropriately interpreted.
FIG. 1.
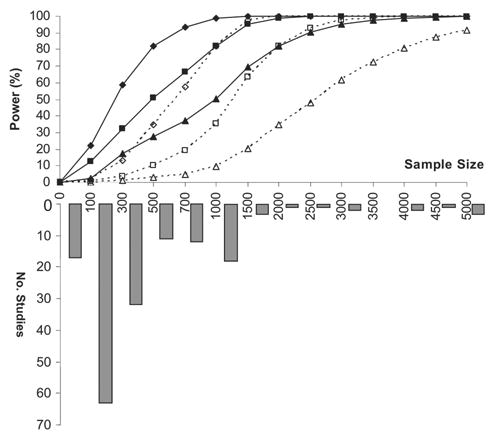
Statistical power for quantitative traits using unrelated population sample, and the sample size distribution of osteoporosis-related association studies published during 2002–2004 (Table 1). In power estimation, QTLs were assumed to be responsible for 2% (♦ or ⋄), 1% (▪ or □), or 0.5% (▴ or ▵) of phenotypic variation, respectively. The power was estimated under an ideal situation, for which the tested marker was the QTL itself with the causal allele frequency of 0.3 and the QTL follows additive inheritance. The significance level was set as α = 0.01 (solid lines) or 0.001 (dashed lines). We set two different α levels because it was suggested that two studies with p < 0.01 or a single study with p < 0.001 is predictive of future replication.(36)
A critical question to be answered is the correlation between osteoporotic fractures (OFs) and osteoporosis risk factors. The search for OF genes should start with significant heritability for OFs and should include risk factors (e.g., BMD) that are genetically correlated with OFs. However, it is unclear to what extent the BMD variation can predict bone fractures, the endpoint of osteoporosis. Studies have suggested that OFs per se can be used as a direct trait for gene hunting of osteoporosis.(131)
MONOGENIC BONE DISEASES/TRAITS AND TRANSGENIC/KNOCKOUT ANIMAL MODELS
A series of candidate genes have been identified by studying monogenic bone diseases/traits. Likewise, using knockout and transgenic animal models, many well-known or novel genes were found to be accountable for abnormal bone phenotypes. Studies on monogenic bone diseases and genetically manipulated animals have provided biologically relevant candidate genes for association studies. They also help isolate and highlight the functions of a particular gene, thus providing further evidence supporting the gene’s relevance to bone mass variation. A salient example is the LRP5 gene. Studies on three monogenic traits, autosomal recessive osteoporosis-pseudoglioma,(132) autosomal dominant high bone mass,(95,133) and autosomal recessive osteopetrosis,(134) have brought to light the LRP5 gene for its potential role in osteoporosis. The first two conditions were proven to result from different mutations of the LRP5 gene,(132,133) and the last one was linked to the genomic region, 11q12–13,(134) which contains the gene. To confirm the significance of LRP5 to bone mass variation in the general population, independent studies were performed to test linkage of 11q12–13 to BMD.(99,135) Transgenic mice expressing a unique mutation (G171V) in LRP5 (which was associated with high bone mass in humans(95,133)) had a similar phenotype with high bone mass and enhanced strength(136) and displayed a role in regulating bone biomechanical properties in mice.(137) More recently, studies have investigated the association of the LRP5 gene polymorphisms with osteoporosis-related phenotypes in various populations.(24,96,97,138–141)
Two review articles(9,10) have comprehensively summarized such studies updated to 2002. Over the past 2 years, 28 new studies were reported, which are summarized in Table 2 (cited online).
GENOME-WIDE LINKAGE STUDIES
Twelve studies were reported during 2003–2004, including 10 whole genome linkage scans(142–151) and 2 follow-up studies(152,153) focusing on candidate regions identified in previous genome scans. The first review(1) summarized several whole genome linkage scan results.(154–158) To substantiate the initial findings and to detect novel loci, several groups performed further studies in expanded samples.(142,147–149,152,153) Efforts were also made to explore confounding factors such as heterogeneity(145) and pleiotropy.(143,146) We outline below some interesting studies classified by the studied phenotypes. The newly identified QTLs are added to Table 3, based on our first review update.(1)
BMD and osteoporotic fractures
Styrkársdóttir et al.(150) reported a whole genome linkage scan in 207 Icelandic osteoporotic families (1323 individuals), using phenotypes combining BMD and osteoporotic fractures. The most significant linkage was found on chromosome 20p12.3 (multipoint LOD = 5.10; p = 6.3 × 10−7). By saturating 30 additional markers on 20p12.3, the region was narrowed down to 6.6 cM. In the follow-up LD mapping and association analyses, they found that three variants in the BMP2 gene, including a missense polymorphism Ser37Ala and two haplotypes (hapB and hapC), were associated with osteoporosis. Depending on phenotypes, Ser37Ala yielded RRs in the range of 3.8–6.3. Ser37Ala and hapC were associated with low BMD and osteoporotic fractures. They further replicated their associations in a Danish cohort of postmenopausal women. The identified associations await confirmation across different ethnic groups, and the biological effects of the three putative variants in the BMP2 gene need to be explored.
In 29 Mexican-American families (664 individuals), Kammerer et al.(144) found a QTL affecting forearm (radius midpoint) BMD on chromosome 4p (LOD = 4.33) and suggestive linkage on 12q (LOD = 2.35). They also found suggestive linkage for trochanter BMD on chromosome 6 (LOD = 2.27). In subgroup analysis for men, they obtained linkage for femoral neck BMD on 2p (LOD = 3.98) and trochanter BMD on 13q (LOD = 3.46).
Shen et al.(149) performed their second whole genome scan in a largely expanded sample (79 pedigrees, 1816 subjects). The sample contained >80,000 relative pairs informative for linkage analysis. The strongest linkage was found on Xq27 with two-point LOD scores of 4.30 for wrist BMD and 2.57 for hip BMD. Linkage was also found on 11q23 for spine BMD (LOD = 3.13), confirming findings in two earlier independent studies.(159,160) Another group performed extension studies(142) to confirm their previous finding on chromosome 1q in 464 premenopausal white sister pairs.(158) They reported replication on chromosome 1q in an independent sample of 254 premenopausal white sister pairs. They further fine mapped (4 cM) the region in all white sister pairs (n = 938) and achieved a LOD score of 4.3. In addition, they tested linkage for hip BMD in 570 white sister pairs and 204 black sister pairs(148) to compare the results with their earlier study.(158) Significant linkage was found on chromosomes 14q (LOD = 3.5 for trochanter BMD) and 15q (LOD = 4.3 for femoral BMD). However, their previous highly significant linkage on 11q12–13(99) disappeared in their follow-up efforts,(142,148) implying that underpowered studies may yield unreliable/unstable results. Another follow-up study(161) was conducted to confirm the linkage on chromosome 3p21 for spine BMD.(162) Thirty additional microsatellite markers within the 3p21 region were genotyped for a cohort of extreme discordant and concordant sib pairs (1098 individuals). The maximum evidence of linkage was LOD 3.6 for age-adjusted spine BMD.
To explore potential pleiotropic effects, Karasik et al.(146) performed principal component analyses in 323 pedigrees from the Framingham Osteoporosis Study. For PC1, loci of suggestive linkage were identified on chromosomes 1q21.3 and 8q24.3 with LOD scores of 2.5 and 2.4, respectively. For PC2, multipoint LOD score was 2.1 on 1p36. Suggestive linkage of PC_hip was found on 8q24.3 and 16p13.2 (LODs > 1.9). The study suggested that QTLs underlying bone mass variation are likely to have pleiotropic effects at different skeletal sites.
To estimate potential heterogeneity of their earlier linkage findings,(156) Karasik et al.(145) performed analyses in subsamples stratified by sex, age, and body mass index (BMI). Heterogeneity was found on chromosomes 6p21.2 and 21qter, where linkage findings in the total sample were not supported in the subsample analyses. However, sub-sample-specific maxima were found on 4q34.1 (males), 9q22–9q31 (younger), 16p13.2 (high BMI), and 17p13.3 (older), which were not reflected by the total sample results. Their results suggested that the genetic effects on bone mass variation could be different between men and women, younger and older, and lean and obese adults. A concern with the study is that subgroup analyses may reduce the statistical power (by reducing sample sizes), although stratified samples could be more homogenous.
Bone structure or bone size
Xu et al.(153) saturated several previously identified regions by genotyping denser markers (~5 cM apart) in an expanded sample of 79 pedigrees. Significant linkage was achieved for wrist bone size on 17q22 (two-point LOD = 2.27, p = 0.0006; multipoint LOD = 1.78, p = 0.002). The chromosomal region 17q22 contains COL1A1, a strong candidate gene associated with osteoporotic fractures.
Another extension study was performed for femoral structures in 437 white and 201 black healthy premenopausal sister pairs,(147) of which 191 white pairs overlapped with their previous sample.(157) Linkage was replicated on chromosomes 3 (LOD = 5.0 for femoral head width; LOD = 3.6 for femur shaft width), 7 (LOD = 5.0 for femoral head width), and 19 (LOD = 3.2 for femoral neck axis length) in the white sister pairs, with a new locus identified on chromosome 8 (LOD = 6.0 for femoral head width). To identify sex-specific loci, they conducted a genome scan in 257 white brother pairs (18–61 years of age) to compare with their sister pairs.(163) The significant linkage was for pelvic axis length (LOD = 4.1) on chromosome 4p. There was no overlap between the linkage regions identified in men and in women, suggesting possible genetic heterogeneity for bone structure. A potential problem of the study is the small sample (257 brother pairs), which may affect validity of the results.(16)
QUS
Wilson et al.(151) performed a genome scan in a cohort of dizygous twin pairs to identify QTLs for bone QUS. Two specific indices, BUA and velocity of sound (VOS), were used as phenotypes. Linkage was found for BUA (LOD 2.1–5.1) on 2q33–37 and for VOS (LOD 2.2–3.4) on 4q12–21. In the Fels Longitudinal Study (453 subjects),(164) linkage was found for calcaneal QUS on chromosome 4p15 near marker D4S419 (LOD = 2.12), a region previously linked to forearm BMD.
Summary
Like many other common complex diseases, replication of linkage results for osteoporosis and related traits has been difficult. Reasons could be multiple: complex etiology of osteoporosis (e.g., genetic heterogeneity, incomplete penetrance, epistasis, variable expressivity, and pleiotropy) and poor study design.(16) However, with the increasing number of studies, the pattern of linkage that is beginning to emerge is encouraging. Not only are there strong linkage signals, but some of these are in genomic regions that harbor prominent positional candidate genes. A more encouraging fact is that we are already beginning to observe replications across studies.(142,149,153) However, caution should be taken in interpretation of replication/confirmation results. Actually, the probability an observed linkage is true depends on the power of initial study and the power of replication study.(16) A high LOD score does not always imply a true linkage, especially when studies are underpowered.
As shown in Table 3, >60 QTLs have been identified from ~20 genome linkage scans. These QTLs were found on all but chromosome Y. Twenty QTLs have been replicated in at least two studies; these QTLs are located on chromosomes 1p36, 1q21–24, 2p21–24, 2q33–37, 3q12–26, 4p15–16, 4q31–34, 5q33–35, 6p21, 8q24-qter, 10q26, 11q23–24, 12q23–24, 13q31–34, 14q12–24, 14q31–32, 16p13, 17p11–13, 19p13-q13, and 21q22-qter. The regions harboring the largest number of replication are 1p36, 1q21–24, 4q31–34, and 12q23–24 in five different studies and 13q31–34 and 17p11–13 in at least three different studies. As the number of linkage studies continues to grow, there is strong reason to anticipate that additional replications will be brought to light. Of course, some genomic regions will eventually be proven to be false positive.
QTL MAPPING IN ANIMALS
QTL mapping in animals, especially in mice, provides a powerful tool to identify human disease genes. It has greatly facilitated the daunting task facing human researchers because of the high homology (~75%) between the human and mouse genomes. In the bone field, some pilot QTL mapping studies in mice include (1) the SAMP6 model of osteopenia by Shimizu et al.(165) and by Bene et al.,(166) which uncovered QTLs on chromosomes 2, 7, 11, 13, and 16 for low bone mass; (2) crosses between C57BL/6J (B6) and DBA/2J (D2) inbred strains by Klein et al.,(160,167) which located whole body BMD QTLs on chromosomes 1, 2, 4, and 11; and (3) crosses between B6 and Castaneus (CAST) or C3H/HeJ (C3H) by Beamer et al.,(168,169) which located femoral BMD QTLs on seven chromosomes.
During 2003–2004, 10 studies were published, reporting new QTLs for BMD, bone structure and strength, bone mechanical properties, and biochemical markers. Klein et al.(170) reported their interesting findings by following a region on mouse chromosome 11 that strongly influenced peak BMD in their earlier study.(167) They generated a DGA/2 (D2) background congenic mouse with an 82-Mb region of chromosome 11 replaced by the corresponding region of the C57BL/6 (B6) genome. The congenic mice had increased peak BMD and improved measures of femoral shaft strength (failure load and stiffness) relative to heterozygous or D2 littermates. Linkage analysis of the congenic B6D2F2 population narrowed the BMD QTL to a 31-Mb region. They next analyzed gene expression in B6 and D2 mice. Microarray analysis of kidney tissue showed that Alox15, which is located in the middle of the identified QTL interval, was the only differentially expressed gene within this chromosomal region. Their studies on Alox15 knockout mice confirmed the role of 12/15-lipoxygenase (12/15-LO) in skeletal development. Pharmacological inhibitors of this enzyme improved BMD and bone strength in two rodent models of osteoporosis. The Alox15 gene encodes 12/15-LO, an enzyme that converts arachidonic and linoleic acids into endogenous ligands for PPAR-γ. Their in vitro observations suggested that genetically determined, constitutively high 12/15-LO expression might limit peak bone mass attainment by suppressing osteogenesis through activation of PPAR-γ–dependent pathways. A concern with the study is that gene expression analysis using kidney tissue revealed a gene relevant to bone metabolism. Whereas the results are exciting, Alox15 is subject to further studies to test with the effects on bone mass variation in the general human population.
A previous interval mapping implicated 12 distinct QTLs for peak femoral BMD in (B6 × C3H)F2 and (B6 × CAST)F2 4-month-old female progeny.(168,169) To test the effect of each QTL, Shultz et al.(171) selected two sets of loci (six each from C3H and CAST) to make congenic strains by repeated backcrossing of donor mice carrying a given QTL-containing chromosomal region to recipient mice of the B6 progenitor strain. In addition, they selected the femoral BMD QTL region on chromosome 1 of C3H for congenic subline development to facilitate fine mapping of this locus. In 11 of 12 congenic strains, 6 B6.C3H and 5 B6.CAST, femoral BMD in mice carrying c3h or cast alleles in the QTL regions was significantly different from that of littermates carrying b6 alleles. Analyses of eight sublines derived from the B6.C3H-1T congenic region revealed two QTLs. Their results indicated that many QTLs identified in the F2 analyses exert independent effects when transferred and expressed in a common genetic background, and decomposition of QTL regions by congenic sublines can reveal additional loci for phenotypes assigned to a QTL and can markedly refine QTLs. Using congenic mice, Turner et al.(172) revealed sex-specific genetic regulation of femoral structure.
An earlier study identified QTLs on mouse chromosomes 1, 4, 6, 13, and 18 for femoral BMD in (B6 × C3H)F2 mice.(169) In a subsequent study,(173) the 999 F2 mouse progeny were phenotyped for measures of femoral biomechanics, structure, and more refined femoral midshaft BMD measures. Two novel multivariate phenotypes were derived using principal component analysis. Results of genome-wide analyses provided strong evidence of pleiotropic effects on chromosome 4. Chromosomes 1, 8, 13, and 14 were found to harbor QTLs affecting phenotypes in two of the three aspects of bone properties. Principal component analysis identified pleiotropic QTLs on chromosomes 4 and 14, influencing nearly all the bone phenotypes.
BMD is not the only risk factor for osteoporosis. Other intermediate phenotypes (e.g., bone structure or size, mechanical properties, and biochemical markers) may offer additional mechanistic insights into the overall processes of peak bone mass acquisition and determinants of bone strength. Volkman et al.(174) studied the genetic determinants of geometric properties of cortical bone (measured by μCT) using a mouse population containing 487 female UM-HET3 mice derived as the progeny of (BALB/cJ × C57BL/6J)F1 females and (C3H/HeJ × DBA/2J)F1 males. Fourteen markers were associated with one or more geometric traits. Because bone strength depends not only on the geometric, but also material properties of the bone, they further exploited QTLs that may affect mechanical and material properties of cortical bone.(175) Femurs from 18-month-old mice were tested to failure in four-point bending to assess mechanical properties of cortical bone. They found QTLs on maternal chromosomes 11 and 13 and paternal chromosomes 2, 4, 7, 10, 11, and 17.
To exploit genetic determinants of vertebral trabecular bone traits, Bouxsein et al.(176) evaluated the fifth lumbar vertebra in 914 adult female mice from the F2 intercross of B6 and C3H progenitor strains. They found a pattern of genetic regulation derived from 13 autosomes, with 5–13 QTLs associated with each of the traits. Using 633 MRL/ SJL F2 mice, Masinde et al.(177) identified nine QTLs underlying periosteal circumference (PC), which accounted for 38.6% of phenotype variance. In addition, four epistatic interactions were found that accounted for 37.6% of phenotype variance. In another study, using a B6.C3H-4T (4T) congenic mouse strain, which is genetically 98.4% B6 and carries the C3H chromosome 4 QTL genomic DNA, Robling et al.(178) found evidence for a skeletal mechanosensitivity gene on mouse chromosome 4. Finally, two studies examined the genetic component for two biochemical markers. Srivastava et al.(179) identified three major QTLs (on chromosomes 2, 6, and 14) that influence blood levels of alkaline phosphatase (ALP) in 518 F2 female mice of MRL/MpJ and SJL progenitor strains. Using 633 F2 female mice (MRL/MpJ × SJL), Mohan et al.(180) revealed six QTLs on chromosomes 1, 9, 10, and 11 for serum IGF binding protein-5 levels.
Summary
QTL mapping in polygenic mice models continues to yield interesting findings. Among these is discovery of Alox15 as a negative regulator of peak BMD in mice,(170) which suggests traditional gene mapping methods combined with functional studies may provide novel understanding of bone mass regulation. A remarkable investment of effort over the past years was on congenic lines, which can be created by repeated backcrossing into one of the parental strains and recombinants thereof. Congenic lines prove to be a useful tool to confirm the QTL existence and fine map the QTLs and to test the quantitative effect of individual QTLs.(171)
FUNCTIONAL GENOMIC STUDIES
Genetic epidemiology studies have provided valuable data for osteoporosis research. However, genetic epidemiology studies, by exploring relationships between genes and osteoporosis or related traits at the DNA level, cannot tell how the genes contribute to the disease. Functional genomic studies, by look for such relationship at the mRNA and protein levels, may help elucidate intermediate biochemical processes of a disease. The goal of functional genomics is not simply to provide a catalog of all the genes and information about their functions, but to understand how the components work together to comprise functioning cells and organisms.(181) Moreover, functional genomic studies may infer novel candidate genes and relevant pathways for DNA-based studies and confirm the results of DNA-based studies by providing functional evidence. DNA microarray and proteomic technologies, which systemically and quantitatively profile the mRNA and protein expression underlying functions of a cell type, tissue, or organisms at the genome level, are important aspects of functional genomics.
DNA microarray studies
Microarray technology allows simultaneous monitoring of gene expression for tens of thousands of genes. In the bone field, DNA microarray was first applied in 1997, when Heller et al.(182) discovered genes involved in rheumatoid arthritis. Since then, microarrays have been applied to study various aspects of osteogenesis and gene expression profiles in osteoporosis tissues. The results derived from these studies are summarized in Table 5 (cited online).
Several studies exploited the orchestrated gene expression during the process of osteoblast differentiation,(183–189) which provided novel insights into the mechanisms of bone formation and mineralization. For example, microarrays were used to determine genes regulated by different factors, such as BMP2 during differentiation of human marrow stromal cells,(190) and Tbx2 in the mouse NIH3T3 cell line.(191) Changes in expression of many genes were reported to occur during differentiation of the mouse calvarial-derived MC3T3-E1 cell line to an osteoblast-like phenotype.(192) Studies have been done to discover genes regulated in bone-related diseases: rheumatoid arthritis,(193–195) ossification of the posterior longitudinal ligament,(196) and osteosarcoma.(197) Human dental pulp stem cells and bone marrow stromal stem cells were found to have a similar level of gene expression for >4000 known human genes, whereas only a few genes were expressed differentially.(198)
Microarray technique has proven to be a powerful tool to find genes for diseases of interest. For example, based on the C2C12-generated expression data set and a training set containing known members of the osteogenic, myoblastic, and adipocytic pathways, 176 new genes were found as relevant to osteogenesis.(199) Gene array analysis of osteoblast differentiation in the mouse calvarial-derived MC3T3-E1 cell line revealed changes that were not anticipated.(192) In a comparative study of gene expression between a congenic strain that contains a QTL of high BMD and its wildtype strain in mice, ~40% of the 8734 studied genes and expressed sequence tags (ESTs) were not documented previously.(200) Gene expression profiles during the mineralization process in bone marrow–derived human mesenchymal stem cells disclosed transcriptional stimulation of 55 genes and repression of 82 genes among >20,000 examined.(201) Among >8700 genes studied in the mouse MC3T3-E1 cell line during osteoblast differentiation, 252 were found to be differentially expressed during the proliferation and mineralization phases.(202)
Thus far, most high-throughput gene expression studies have used cultured cell lines of humans, mice, or rats. Using cell cultures has certain advantages, such as virtually no limit to tissue supply, high purity, and homogeneity of desired cell populations, possibility to easily modify experimental conditions. However, it may pose potential problems to the results obtained. Even primary cultures of stromal cells from human bone marrow gradually lose their osteogenic potential,(203,204) which may suggest changes in gene expression profiles. Moreover, cultured tissues lack relationships with other tissues. For complex disease research, it is a significant shortcoming, because it eliminates the influence of genes and/or other factors, which are not expressed in the diseased tissues, but contribute to their development. This may introduce a significant bias into gene expression profiles of cultured cells compared with freshly isolated ones. Given that, studying fresh bone or bone-related tissues may be a promising way to obtain closer to reality data on expression profiles for genes that are tissue specific and differentially expressed locally.
Bone marrow is heterogeneous in terms of cell composition and consists of many cell types, which have a potential to differentiate into various cell lineages. Some cell types of interest, such as bone marrow mesenchymal stem cells, which can differentiate into osteoblastic lineage, comprise only a minor portion of the whole bone marrow cell population. Although bone marrow cell lineages may be ideal for microarray studies, there are currently technical difficulties related to the isolation of the sufficient number of intact cells and, subsequently, RNA for the analysis. Studying gene expression profiles in monocyte/macrophage lineage cells may be of interest, because these cells are early progenitors of osteoclasts and produce cytokines important to bone metabolism,(205) and it is practical to isolate sufficient RNA from circulating monocytes for microarray analysis. Liu et al.(206) reported an in vivo study in humans using circulating monocyte. They performed comparative gene expression studies for circulating monocytes in 10 subjects with high versus 9 with low BMD(206) and identified 66 differentially expressed genes. After real-time RT-PCR validation, they found three very interesting genes, CCR3 (chemokine receptor 3), HDC (histidine decarboxylase), and GCR (glucocorticoid receptor), to be upregulated in subjects with lower BMD. The results suggested a novel pathophysiology mechanism for osteoporosis that is characterized by increased bone monocyte recruitment, increased monocyte differentiation into osteoclasts, and increased osteoclast stimulation through monocyte functional changes. The study provided helpful information for future research using fresh tissue samples such as bone marrow cells that include precursors for osteoblast lineages.
Recently developments in cell and molecular biology make it possible to obtain a genome-wide expression profile of a single cell.(207) Single cell gene expression analysis is currently used to generate data within the fundamental unit, the single cell, thereby freeing the analysis from assumptions or questions regarding cell population homogeneity. The data obtained with this approach suggest that even morphologically identical cells may have quite distinct transcriptomes, which gives deeper insights into cell differentiation and functional assignment.(208,209) Given that the processes of osteogenesis and bone metabolism are multistage and involve many cell types (osteogenic precursors, various bone cellular components, T and B cells, etc.), including those that are able to transdifferentiate, this approach may be useful for fine characterization of the distinct bone-related cell types. Single cell expression profiling may also offer a highly parallel view of the workings of a gene regulatory network at one specific point in time and will hopefully provide insights that could lead to an improved ability to interpret gene expression patterns. However, its direct relevance and significance in relation to disease gene identification (e.g., osteoporosis) remains to be determined.
Advances in functional genomics and computer sciences make it possible to analyze the gene expression changes in the context of known biological pathways.(184,185,187) However, many challenges remain in the use of functional genomic approach for complex bone disorders. The questions to be answered include how various environmental, lifestyles or inherent regulating factors contribute to the BMD variation, onset, and development of osteoporosis, and risk of osteoporotic fractures. Another question is identification of relationships and interaction between genes that have been reported to be either associated with or linked to disorders. Do their expression levels correlate with the degree of association or linkage? Do these genes interact in developing the trait?
Proteomics studies
Because of complicated processes such as alternative mRNA splicing and post-translational modifications, the correlation between mRNA and protein expression level(184,185,210–213) could be low. There are ~30,000 genes in the human genome, but the estimated number of proteins in human cells is between ~300,000 and ~1,000,000.(214) Thus, the proteomics approach, complementary to DNA microarrays, is an indispensable component of functional genomics.
Proteomics can be classified into three major types: expression proteomics, functional proteomics, and structural proteomics.(215) Expression proteomics quantitatively analyzes and identifies differentially expressed proteins from protein profiles between case and control groups. Those differentially expressed proteins provide biomarkers or important proteins in pathways underlying different biomedical conditions. Functional proteomics involves the global understanding of protein–protein interactions. Because key proteins involved in disease development generally interact with other proteins, functional proteomics is a favorable approach for unveiling whole pathways participating in the etiology of diseases. To better understand and even predict protein function, one should figure out 3D structures of the proteome. Structural proteomics may prospectively fulfill this goal by mapping out the structures of protein complexes or the proteins in a specific cellular organelle.(216)
Application of proteomics in the bone field has a relatively short history, and more results from their use are yet to come. Applying an expression proteomics approach on cultured cells, pilot studies identified some novel proteins important for the development of bone marrow hemato-poietic cells,(217) mesenchymal chondroblasts,(218) and osteoblasts.(219) Combining 2-dimensional electrophoresis (2-DE) and isotype coded affinity tag (ICAT) techniques, a study suggested novel proteins related to osteoclast differentiation.(220) A proteomics approach was used in seeking inhibitors of osteoclast-mediated bone resorption and is currently screening for bone anabolic agents.(221) Some in vivo studies were performed to characterize global scale molecular profiling of a variety of bone-related diseases.(222) For example, a study showed comparative molecular characterization at the transcriptome (microarray with 12,526 gene specificities) and proteome levels (multi-Western blot PowerBlot with 791 antibodies) of synovial tissue from patients with rheumatoid arthritis (RA) compared with those with osteoarthritis (OA).(222) Several new candidate molecules (e.g., Cathepsin D and Stat1) displayed reproducible differences of expression in patients with RA versus patients with OA.
Proteomics represents one of the most promising fields poised to boost our understanding of systems level cellular behavior and the fundamental etiology of osteoporosis that can be related to specific genes. It is anticipated that a huge amount of data will be produced for years to come, and the wealth of information will undoubtedly benefit osteoporosis research communities
SUMMARY
Since our first update by the end of 2002, remarkable progress has been made in revealing the molecular genetic basis of osteoporosis. The numbers of QTLs, genes, and other markers linked and/or associated with osteoporosis-related traits continue to expand and become significantly more detailed and complex. There are now several promising chromosomal regions and candidate genes that are supported by multiple studies. On the other hand, the majority of findings are still inconclusive pending further study, which calls for new approaches and strategies with both sensitivity and robustness to accommodate confounding effects from various sources. With the rapid development in human and model organisms genome sequences, and the progress in molecular technologies, analytical tools, bioinformatics, and functional genomics, one can expect that it will be possible to define the genes and mutations and their functions in the predisposition or the resistance to osteoporosis.
Acknowledgments
HWD was partially supported by NIH and the State of Nebraska (LB595). The study was also supported by grants from CNSF, Huo Ying Dong Education Foundation, the Ministry of Education of China, and Xi’An Jiao Tong University.
APPENDIX: GLOSSARY
- Allelic heterogeneity
The same phenotypic outcome can be caused by different variants within the same gene. For example, vitamin D-dependent rickets type IIA could result from any of eight different mutations in the vitamin D receptor (VDR) gene.
- Association analysis
Population-based or family-based genetic studies that examine whether an allele of a certain gene or marker co-occurs with a phenotype (e.g., a disease) at a significantly higher rate than predicted by chance alone.
- Complex trait
A measured phenotype, such as disease status or a quantitative character, which is influenced by many environmental and genetic factors, and potentially by interactions among them.
- Effect-size estimates
Tests of a null hypothesis can show that an effect is significant but not how large the effect is. Measures of effect size are based on the proportion of variance in the data that can be attributed to the experimental variables.
- Epistasis
A form gene interaction whereby one gene masks or interferes with the phenotypic expression of one or more genes at other loci.
- Gene-environment interaction
A phenomenon whereby an individuals’ genotype interacts with environment factors to determine his/her risk of disease. In practice, this interaction is shown as that the effect of a variant manifests only in populations with a certain environmental exposure.
- Genomic control
A method to assess population stratification by using the independent marker loci to adjust the distribution of a standard test statistic.
- Haplotype
A combination of alleles at different sites on a single chromosome.
- Heritability
The proportion of the phenotypic variance caused by genetic variance. It reflects the degree to which the phenotype is inherited.
- Linkage analysis
the analysis of family-based genotipic data to detect cosegregation of a disease locus with one or more loci within a family.
- Linkage disequilibrium (LD)
Two loci that are in linkage disequilibrium are inherited together more often than would be expected by chance. LD depends heavily on population history.
- Locus heterogeneity
The same phenotypic outcome can be caused by variants at different genetic loci. For example, alleles at both the BRCA1 and BRCA2 locus can increase susceptibility to breast cancer.
- LOD (log of the odds) score
A measure of the likelihood of genetic linkage between loci. The log (base 10) of the odds that the loci are linked (with recombination fraction θ) rather than unlinked. In classical genetics, a LOD score greater than +3 is evidence of significant linkage; one that is greater than +1.9 is evidence of suggestive linkage.
- Odds ratio (OR)
This is closely related to the relative risk and is defined as the odds of possessing the phenotype in those with the variant allele divided by the odds of possessing the phenotype in those without the variant allele. Odds ratios are simply a different way of expressing this association than relative risk because they compare odds rather than risk of an event.
- Penetrance
The probability of an individual expresses the character of a trait/disease in the phenotype, given that he/she has a certain genotype. If the phenotype is always expressed in the presence of the genotype, the genotype is completely penetrant. If it is not always expressed, it is incompletely penetrant.
- Pleiotropy
A genetic variant can affect more than one trait.
- Population stratification
The presence of multiple subgroups with different allele frequencies within a population. The different underlying allele frequencies in sampled subgroups might be independent of the disease within each group, and they can lead to erroneous conclusions of linkage disequilibrium or disease relevance.
- Power
The power of a statistical test is the probability that the test will correctly reject the null hypothesis when it is false. The higher the power, the greater the chance of obtaining a statistical significant result when the null hypothesis is false.
- Relative risk (RR)
The ratio of the incidence of the phenotype under consideration in subjects with the variant allele to the incidence in those without the variant allele.
- Quantitative trait loci (QTL)
Genetic loci where allelic variation is associated with variation in a quantitative trait.
- Structured association
A method to infer the details of population structure to testing for association.
- Transmission disequilibrium test (TDT)
A method of detecting genetic association that avoids problems of population stratification. Instead of comparing unrelated cases and controls, the test determines whether given the parental genotypes, the alleles that are transmitted from parent to child and the child’s affectation status are independent.
- Type I error
The probability of rejecting the null hypothesis when it is true. For association or linkage studies, type I errors are manifest as false-positive reports.
Footnotes
The authors state that they have no conflicts of interest.
References
- 1.Liu YZ, Liu YJ, Recker RR, Deng HW. Molecular studies of identification of genes for osteoporosis: The 2002 update. J Endocrinol. 2003;177:147–196. doi: 10.1677/joe.0.1770147. [DOI] [PubMed] [Google Scholar]
- 2.Blank RD. Breaking down bone strength: A perspective on the future of skeletal genetics. J Bone Miner Res. 2001;16:1207–1211. doi: 10.1359/jbmr.2001.16.7.1207. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TV, Eisman JA. Genetics of fracture: Challenges and opportunities. J Bone Miner Res. 2000;15:1253–1256. doi: 10.1359/jbmr.2000.15.7.1253. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TV, Blangero J, Eisman JA. Genetic epidemiological approaches to the search for osteoporosis genes. J Bone Miner Res. 2000;15:392–401. doi: 10.1359/jbmr.2000.15.3.392. [DOI] [PubMed] [Google Scholar]
- 5.Audi L, Garcia-Ramirez M, Carrascosa A. Genetic determinants of bone mass. Horm Res. 1999;51:105–123. doi: 10.1159/000023343. [DOI] [PubMed] [Google Scholar]
- 6.Baldock PA, Eisman JA. Genetic determinants of bone mass. Curr Opin Rheumatol. 2004;16:450–456. doi: 10.1097/01.moo.0000127828.34643.b4. [DOI] [PubMed] [Google Scholar]
- 7.Eisman JA. Genetics of osteoporosis. Endocr Rev. 1999;20:788–804. doi: 10.1210/edrv.20.6.0384. [DOI] [PubMed] [Google Scholar]
- 8.Huang QY, Recker RR, Deng HW. Searching for osteoporosis genes in the post-genome era: Progress and challenges. Osteoporos Int. 2003;14:701–715. doi: 10.1007/s00198-003-1445-9. [DOI] [PubMed] [Google Scholar]
- 9.Peacock M, Turner CH, Econs MJ, Foroud T. Genetics of osteoporosis. Endocr Rev. 2002;23:303–326. doi: 10.1210/edrv.23.3.0464. [DOI] [PubMed] [Google Scholar]
- 10.Ralston SH. Genetic determinants of susceptibility to osteoporosis. Curr Opin Pharmacol. 2003;3:286–290. doi: 10.1016/s1471-4892(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 11.Rizzoli R, Bonjour JP, Ferrari SL. Osteoporosis, genetics and hormones. J Mol Endocrinol. 2001;26:79–94. doi: 10.1677/jme.0.0260079. [DOI] [PubMed] [Google Scholar]
- 12.Stewart TL, Ralston SH. Role of genetic factors in the pathogenesis of osteoporosis. J Endocrinol. 2000;166:235–245. doi: 10.1677/joe.0.1660235. [DOI] [PubMed] [Google Scholar]
- 13.Zmuda JM, Cauley JA, Ferrell RE. Recent progress in understanding the genetic susceptibility to osteoporosis. Genet Epidemiol. 1999;16:356–367. doi: 10.1002/(SICI)1098-2272(1999)16:4<356::AID-GEPI3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Recker RR, Deng HW. Molecular and genetic mechanisms of osteoporosis: Implication for treatment. Curr Mol Med. 2003;3:737–757. doi: 10.2174/1566524033479375. [DOI] [PubMed] [Google Scholar]
- 15.Dvornyk V, Shen H, Liu YJ, Xiao P, Recker R, Deng HW. Systematic approaches to the study of complex bone disorders at whole-genome level. Curr Genomics. 2004;5:93–108. [Google Scholar]
- 16.Shen H, Liu YJ, Liu PY, Recker RR, Deng HW. Non-replication in genetic studies of complex diseases—lessons learned from studies of osteoporosis and tentative remedies. J Bone Miner Res. 2005;20:365–376. doi: 10.1359/JBMR.041129. [DOI] [PubMed] [Google Scholar]
- 17.Gorai I, Inada M, Morinaga H, Uchiyama Y, Yamauchi H, Chaki O, Hirano H. Cytochrome P450c17α(CYP17) gene polymorphism indirectly influences on bone density through their effects on endogenous androgen in postmenopausal Japanese women-Are the effects of age and body mass index greater than those of endogenous sex steroids? J Bone Miner Res. 2004;19:S382. [Google Scholar]
- 18.Napoli N, Mumm S, Sheik S, Rini GB, Villareal RC. Effect of CYP450 gene polymorphisms on estrogen metabolism and bone density. J Bone Miner Res. 2004;19:S384. doi: 10.1359/JBMR.041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezura Y, Nakajima T, Kajita M, Ishida R, Inoue S, Yoshida H, Suzuki T, Shiraki M, Hosoi T, Orimo H, Emi M. Association of molecular variants, haplotypes, and linkage disequilibrium within the human vitamin D-binding protein (DBP) gene with postmenopausal bone mineral density. J Bone Miner Res. 2003;18:1642–1649. doi: 10.1359/jbmr.2003.18.9.1642. [DOI] [PubMed] [Google Scholar]
- 20.Dennison EM, Syddall HE, Rodriguez S, Voropanov A, Day IN, Cooper C. Polymorphism in the growth hormone gene, weight in infancy, and adult bone mass. J Clin Endocrinol Metab. 2004;89:4898–4903. doi: 10.1210/jc.2004-0151. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki H, Emi M, Ezura Y, Ishida R, Kajita M, Kodaira M, Yoshida H, Suzuki T, Hosoi T, Inoue S, Shiraki M, Swensen J, Orimo H. Association of a Trp16Ser variation in the gonadotropin releasing hormone signal peptide with bone mineral density, revealed by SNP-dependent PCR typing. Bone. 2003;32:185–190. doi: 10.1016/s8756-3282(02)00949-3. [DOI] [PubMed] [Google Scholar]
- 22.Langdahl BL, Husted LB, Stenkjaer L, Carstens M. Polymorphisms in the IGF-II gene are associated with body weight and bone mass. J Bone Miner Res. 2004;19:S385. [Google Scholar]
- 23.Koh JM, Kim DJ, Hong JS, Park JY, Lee KU, Kim SY, Kim GS. Estrogen receptor alpha gene polymorphisms Pvu II and Xba I influence association between leptin receptor gene polymorphism (Gln223Arg) and bone mineral density in young men. Eur J Endocrinol. 2002;147:777–783. doi: 10.1530/eje.0.1470777. [DOI] [PubMed] [Google Scholar]
- 24.Boot AM, Wilson SG, Dick IM, Islam AFM, Ueland T, Devine A, Prince RL. LRP5 gene polymorphisms predict bone mass and incident fractures in elderly Australian women. J Bone Miner Res. 2004;19:S383. doi: 10.1016/j.bone.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Ando F, Niino N, Shimokata H. Association of polymorphisms of interleukin-6, osteocalcin, and vitamin D receptor genes, alone or in combination, with bone mineral density in community-dwelling Japanese women and men. J Clin Endocrinol Metab. 2003;88:3372–3378. doi: 10.1210/jc.2002-021449. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Ando F, Niino N, Shimokata H. Association of a polymorphism of the CC chemokine receptor-2 gene with bone mineral density. Genomics. 2002;80:8–12. doi: 10.1006/geno.2002.6793. [DOI] [PubMed] [Google Scholar]
- 27.Kornak U, Branger S, Ostertag A, de Vernejoul MC. A VNTR in the CLCN7 gene influences bone density in patients with autosomal dominant osteopetrosis (ADO) type II and in post-menopausal women. J Bone Miner Res. 2004;19:S387. [Google Scholar]
- 28.Eriksson A, Lorentzon M, Anderson N, Mellstrom D, Ohlsson C. The catechol-O-methyltransferase val158met polymorphism is associated with bone mineral density in young adult men. J Bone Miner Res. 2004;19:S131. doi: 10.1359/JBMR.040909. [DOI] [PubMed] [Google Scholar]
- 29.Giraudeau FS, McGinnis RE, Gray IC, O’Brien EJ, Doncaster KE, Spurr NK, Ralston SH, Reid DM, Wood J. Characterization of common genetic variants in cathepsin K and testing for association with bone mineral density in a large cohort of perimenopausal women from Scotland. J Bone Miner Res. 2004;19:31–41. doi: 10.1359/JBMR.0301205. [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y, Ando F, Niino N, Shimokata H. Association of a polymorphism of the dopamine receptor D4 gene with bone mineral density in Japanese men. J Hum Genet. 2003;48:629–633. doi: 10.1007/s10038-003-0090-7. [DOI] [PubMed] [Google Scholar]
- 31.Ishida R, Ezura Y, Emi M, Kajita M, Yoshida H, Suzuki T, Hosoi T, Inoue S, Shiraki M, Ito H, Orimo H. Association of a promoter haplotype (−1542G/−525C) in the tumor necrosis factor receptor associated factor-interacting protein gene with low bone mineral density in Japanese women. Bone. 2003;33:237–241. doi: 10.1016/s8756-3282(03)00226-6. [DOI] [PubMed] [Google Scholar]
- 32.Enattah N, Valimaki VV, Valimaki MJ, Loyttyniemi E, Sahi T, Jarvela I. Molecularly defined lactose malabsorption, peak bone mass and bone turnover rate in young Finnish men. Calcif Tissue Int. 2004;75:488–493. doi: 10.1007/s00223-004-0029-9. [DOI] [PubMed] [Google Scholar]
- 33.Joseph C, Prestwood KM, Burleson JA, Gregersen PK, Lee A, Bucala R, Kuchel GA, Kenny AM. Macrophage migration inhibitory factor (MIF) gene promoter region polymorphism and femoral neck bone density. J Bone Miner Res. 2004;19:S130–S131. [Google Scholar]
- 34.Yamada Y, Ando F, Niino N, Shimokata H. Association of a polymorphism of the matrix metalloproteinase-1 gene with bone mineral density. Matrix Biol. 2002;21:389–392. doi: 10.1016/s0945-053x(02)00030-6. [DOI] [PubMed] [Google Scholar]
- 35.Yamada Y, Ando F, Niino N, Shimokata H. Association of a polymorphism of the matrix metalloproteinase-9 gene with bone mineral density in Japanese men. Metabolism. 2004;53:135–137. doi: 10.1016/j.metabol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Zmuda JM, Cauley JA, Ferrel RE. Nuclear receptor coativator-3 (NCO A3/AIB1/SRC3) alleles are a strong correlate of bioavailable testosterone and vertebral bone mass in older men. J Bone Miner Res. 2004;19:S387. [Google Scholar]
- 37.Heikkinen AM, Niskanen LK, Salmi JA, Koulu M, Pesonen U, Uusitupa MI, Komulainen MH, Tuppurainen MT, Kroger H, Jurvelin J, Saarikoski S. Leucine7 to proline7 polymorphism in prepro-NPY gene and femoral neck bone mineral density in postmenopausal women. Bone. 2004;35:589–594. doi: 10.1016/j.bone.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Koh J, Park E, Kim G, Shin H, Kim S. Polymorphisms in the osteoclast-associated receptor gene are associated with risk of osteopenia and osteoporosis. J Bone Miner Res. 2004;19:S384. [Google Scholar]
- 39.Spotila LD, Rodriguez H, Koch M, Tenenhouse HS, Tenen-house A, Li H, Devoto M. Association analysis of bone mineral density and single nucleotide polymorphisms in two candidate genes on chromosome 1p36. Calcif Tissue Int. 2003;73:140–146. doi: 10.1007/s00223-002-2079-1. [DOI] [PubMed] [Google Scholar]
- 40.Yamada Y, Ando F, Niino N, Miki T, Shimokata H. Association of polymorphisms of paraoxonase 1 and 2 genes, alone or in combination, with bone mineral density in community-dwelling Japanese. J Hum Genet. 2003;48:469–475. doi: 10.1007/s10038-003-0063-x. [DOI] [PubMed] [Google Scholar]
- 41.Omasu F, Ezura Y, Kajita M, Ishida R, Kodaira M, Yoshida H, Suzuki T, Hosoi T, Inoue S, Shiraki M, Orimo H, Emi M. Association of genetic variation of the RIL gene, encoding a PDZ-LIM domain protein and localized in 5q31.1, with low bone mineral density in adult Japanese women. J Hum Genet. 2003;48:342–345. doi: 10.1007/s10038-003-0035-1. [DOI] [PubMed] [Google Scholar]
- 42.Haney EM, Marshall L, Lambert L, Zmuda J, Stone K, Orwoll E, Bliziotes M. An intron 2 polymorphism at the serotonin transporter is associated with reduced hip bone mineral density. J Bone Miner Res. 2004;19:S131. [Google Scholar]
- 43.Balemans W, Foernzler D, Parsons C, Ebeling M, Thompson A, Reid DM, Lindpaintner K, Ralston SH, Van Hul W. Lack of association between the SOST gene and bone mineral density in perimenopausal women: Analysis of five polymorphisms. Bone. 2002;31:515–519. doi: 10.1016/s8756-3282(02)00844-x. [DOI] [PubMed] [Google Scholar]
- 44.Sobacchi C, Vezzoni P, Reid DM, McGuigan FE, Frattini A, Mirolo M, Albhaga OM, Musio A, Villa A, Ralston SH. Association between a polymorphism affecting an AP1 binding site in the promoter of the TCIRG1 gene and bone mass in women. Calcif Tissue Int. 2004;74:35–41. doi: 10.1007/s00223-002-0004-2. [DOI] [PubMed] [Google Scholar]
- 45.Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Arai H, Miyamoto KI, Yoshida M, Yamamoto H, Taketani Y, Morita K, Kubota M, Yoshida S, Ikeda M, Watabe F, Kanemasa Y, Takeda E. The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J Bone Miner Res. 2001;16:1256–1264. doi: 10.1359/jbmr.2001.16.7.1256. [DOI] [PubMed] [Google Scholar]
- 47.Morita A, Iki M, Dohi Y, Ikeda Y, Kagamimori S, Kagawa Y, Matsuzaki T, Yoneshima H, Marumo F. Prediction of bone mineral density from vitamin D receptor polymorphisms is uncertain in representative samples of Japanese Women. The Japanese Population-based Osteoporosis (JPOS) Study. Int J Epidemiol. 2004;33:979–988. doi: 10.1093/ije/dyh245. [DOI] [PubMed] [Google Scholar]
- 48.Fang Y, van Meurs JB, Bergink AP, Hofman A, van Duijn CM, van Leeuwen JP, Pols HA, Uitterlinden AG. Cdx-2 polymorphism in the promoter region of the human vitamin D receptor gene determines susceptibility to fracture in the elderly. J Bone Miner Res. 2003;18:1632–1641. doi: 10.1359/jbmr.2003.18.9.1632. [DOI] [PubMed] [Google Scholar]
- 49.Thakkinstian A, D’Este C, Eisman J, Nguyen T, Attia J. Meta-analysis of molecular association studies: Vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Miner Res. 2004;19:419–428. doi: 10.1359/JBMR.0301265. [DOI] [PubMed] [Google Scholar]
- 50.Thakkinstian A, D’Este C, Attia J. Haplotype analysis of VDR gene polymorphisms: A meta-analysis. Osteoporos Int. 2004;15:729–734. doi: 10.1007/s00198-004-1601-x. [DOI] [PubMed] [Google Scholar]
- 51.Morrison N. Commentary: Vitamin D receptor polymorphism and bone mineral density: Effect size in Caucasians means detection is uncertain in small studies. Int J Epidemiol. 2004;33:989–994. doi: 10.1093/ije/dyh367. [DOI] [PubMed] [Google Scholar]
- 52.Fang Y, van Meurs J, Zhao HY, van Leeuwen J, Pols H, Utterlinden A. Intragenic interaction of VDR haplotype alleles determines fracture risk. J Bone Miner Res. 2004;19:S385. [Google Scholar]
- 53.Nejentsev S, Godfrey L, Snook H, Rance H, Nutland S, Walker NM, Lam AC, Guja C, Ionescu-Tirgoviste C, Undlien DE, Ronningen KS, Tuomilehto-Wolf E, Tuomilehto J, Newport MJ, Clayton DG, Todd JA. Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet. 2004;13:1633–1639. doi: 10.1093/hmg/ddh169. [DOI] [PubMed] [Google Scholar]
- 54.van Meurs J, Zillikens C, Fang Y, Zhao H, van Leeuwen J, Hofman A, Obermayer-Pietsch B, Pols H, Utterlinden A. A DNA variant correlated to lactose intolerance Interacts with VDR gene variants to determine height, bone geometry and BMD. J Bone Miner Res. 2004;19:S385. [Google Scholar]
- 55.Clark AG. The role of haplotypes in candidate gene studies. Genet Epidemiol. 2004;27:321–333. doi: 10.1002/gepi.20025. [DOI] [PubMed] [Google Scholar]
- 56.Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- 57.Yamada Y, Ando F, Niino N, Ohta S, Shimokata H. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density of the femoral neck in elderly Japanese women. J Mol Med. 2002;80:452–460. doi: 10.1007/s00109-002-0348-0. [DOI] [PubMed] [Google Scholar]
- 58.Zhao LJ, Liu PY, Long JR, Lu Y, Xu FH, Zhang YY, Shen H, Xiao P, Elze L, Recker RR, Deng HW. Test of linkage and/or association between the estrogen receptor alpha gene with bone mineral density in Caucasian nuclear families. Bone. 2004;35:395–402. doi: 10.1016/j.bone.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 59.Qin YJ, Shen H, Huang QR, Zhao LJ, Zhou Q, Li MX, He JW, Mo XY, Lu JH, Recker RR, Deng HW. Estrogen receptor alpha gene polymorphisms and peak bone density in Chinese nuclear families. J Bone Miner Res. 2003;18:1028–1035. doi: 10.1359/jbmr.2003.18.6.1028. [DOI] [PubMed] [Google Scholar]
- 60.van Meurs JB, Schuit SC, Weel AE, van der Klift M, Bergink AP, Arp PP, Colin EM, Fang Y, Hofman A, van Duijn CM, van Leeuwen JP, Pols HA, Uitterlinden AG. Association of 5′ estrogen receptor alpha gene polymorphisms with bone mineral density, vertebral bone area and fracture risk. Hum Mol Genet. 2003;12:1745–1754. doi: 10.1093/hmg/ddg176. [DOI] [PubMed] [Google Scholar]
- 61.Ioannidis J, Ralston S, Bennett S, Brandi M, Grinberg D, Karassa F, Langdahl B, van Meurs J, Mosekilde L, Scollen S, Albagha O, Bustamante M, Carey A, Dunning A, Enjuanes A, van Leeuwen J, Mavilia C, Masi L, McGuigan F, Nogues X, Pols H, Reid D, Schuit S, Sherlock R, Utterlinden A. Large-scale evidence for differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes: The GENO-MOS study. J Bone Miner Res. 2004;292:2105–2114. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- 62.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–182. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 63.Gerdhem P, Brandstrom H, Stiger F, Obrant K, Melhus H, Ljunggren O, Kindmark A, Akesson K. Association of the collagen type 1 (COL1A 1) Sp1 binding site polymorphism to femoral neck bone mineral density and wrist fracture in 1044 elderly Swedish women. Calcif Tissue Int. 2004;74:264–269. doi: 10.1007/s00223-002-2159-2. [DOI] [PubMed] [Google Scholar]
- 64.Zhang YY, Lei SF, Mo XY, Wang YB, Li MX, Deng HW. The 1997 G/T polymorphism in the COLIA1 upstream regulatory region is associated with hip bone mineral density (BMD) in Chinese nuclear families. Calcif Tissue Int. 2005;76:107–112. doi: 10.1007/s00223-004-0110-4. [DOI] [PubMed] [Google Scholar]
- 65.Long JR, Liu PY, Lu Y, Xiong DH, Zhao LJ, Zhang YY, Elze L, Recker RR, Deng HW. Association between COL1A1 gene polymorphisms and bone size in Caucasians. Eur J Hum Genet. 2004;12:383–388. doi: 10.1038/sj.ejhg.5201152. [DOI] [PubMed] [Google Scholar]
- 66.Mann V, Ralston SH. Meta-analysis of COL1A1 Sp1 polymorphism in relation to bone mineral density and osteoporotic fracture. Bone. 2003;32:711–717. doi: 10.1016/s8756-3282(03)00087-5. [DOI] [PubMed] [Google Scholar]
- 67.Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP, Aspden RM, Ralston SH. A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest. 2001;107:899–907. doi: 10.1172/JCI10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The International HapMap Project. Nature . 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 69.Passaro A, Vanini A, Calzoni F, Alberti L, Zamboni PF, Fellin R, Solini A. Plasma homocysteine, methylenetetrahydrofolate reductase mutation and carotid damage in elderly healthy women. Atherosclerosis. 2001;157:175–180. doi: 10.1016/s0021-9150(00)00696-1. [DOI] [PubMed] [Google Scholar]
- 70.Hak AE, Polderman KH, Westendorp IC, Jakobs C, Hofman A, Witteman JC, Stehouwer CD. Increased plasma homocysteine after menopause. Atherosclerosis. 2000;149:163–168. doi: 10.1016/s0021-9150(99)00321-4. [DOI] [PubMed] [Google Scholar]
- 71.Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, Rozen R. Human methylenetetrahydrofolate reductase: Isolation of cDNA mapping and mutation identification. Nat Genet. 1994;7:551. [PubMed] [Google Scholar]
- 72.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 73.Miyao M, Morita H, Hosoi T, Kurihara H, Inoue S, Hoshino S, Shiraki M, Yazaki Y, Ouchi Y. Association of methylenetetrahydrofolate reductase (MTHFR) polymorphism with bone mineral density in postmenopausal Japanese women. Calcif Tissue Int. 2000;66:190–194. doi: 10.1007/s002230010038. [DOI] [PubMed] [Google Scholar]
- 74.Abrahamsen B, Madsen JS, Tofteng CL, Stilgren L, Bladbjerg EM, Kristensen SR, Brixen K, Mosekilde L. A common methylenetetrahydrofolate reductase (C677T) polymorphism is associated with low bone mineral density and increased fracture incidence after menopause: Longitudinal data from the Danish osteoporosis prevention study. J Bone Miner Res. 2003;18:723–729. doi: 10.1359/jbmr.2003.18.4.723. [DOI] [PubMed] [Google Scholar]
- 75.Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996;93:7 –9. doi: 10.1161/01.cir.93.1.7. [DOI] [PubMed] [Google Scholar]
- 76.McLean RR, Karasik D, Selhub J, Tucker KL, Ordovas JM, Russo GT, Cupples LA, Jacques PF, Kiel DP. Association of a common polymorphism in the methylenetetrahydrofolate reductase (MTHFR) gene with bone phenotypes depends on plasma folate status. J Bone Miner Res. 2004;19:410–418. doi: 10.1359/JBMR.0301261. [DOI] [PubMed] [Google Scholar]
- 77.Macdonald HM, McGuigan FE, Fraser WD, New SA, Ralston SH, Reid DM. Methylenetetrahydrofolate reductase polymorphism interacts with riboflavin intake to influence bone mineral density. Bone. 2004;35:957–964. doi: 10.1016/j.bone.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 78.Macdonald HM, McGuigan FE, Ralston SH, Reid DM. Association of apolipoprotein E genotypes with bone mineral density and bone loss in early postmenopausal Scottish women: No evidence of gene nutrient interaction with dietary vitamin K or fat. J Bone Miner Res. 2004;19:S422–S423. [Google Scholar]
- 79.Long JR, Liu PY, Liu YJ, Lu Y, Shen H, Zhao LJ, Xiong DH, Deng HW. APOE haplotypes influence bone mineral density in Caucasian males but not females. Calcif Tissue Int. 2004;75:299–304. doi: 10.1007/s00223-004-0034-z. [DOI] [PubMed] [Google Scholar]
- 80.Janssen JA, Burger H, Stolk RP, Grobbee DE, de Jong FH, Lamberts SW, Pols HA. Gender-specific relationship between serum free and total IGF-I and bone mineral density in elderly men and women. Eur J Endocrinol. 1998;138:627–632. doi: 10.1530/eje.0.1380627. [DOI] [PubMed] [Google Scholar]
- 81.Kurland ES, Rosen CJ, Cosman F, McMahon D, Chan F, Shane E, Lindsay R, Dempster D, Bilezikian JP. Insulin-like growth factor-I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1997;82:2799–2805. doi: 10.1210/jcem.82.9.4253. [DOI] [PubMed] [Google Scholar]
- 82.Rivadeneira F, Houwing-Duistermaat JJ, Vaessen N, Vergeer-Drop JM, Hofman A, Pols HA, van Duijn CM, Uitterlinden AG. Association between an insulin-like growth factor I gene promoter polymorphism and bone mineral density in the elderly: The Rotterdam Study. J Clin Endocrinol Metab. 2003;88:3878–3884. doi: 10.1210/jc.2002-021813. [DOI] [PubMed] [Google Scholar]
- 83.Rivadeneira F, Houwing-Duistermaat JJ, Beck TJ, Janssen JA, Hofman A, Pols HA, van Duijn CM, Uitterlinden AG. The influence of an insulin-like growth factor I gene promoter polymorphism on hip bone geometry and the risk of nonvertebral fracture in the elderly: The Rotterdam Study. J Bone Miner Res. 2004;19:1280–1290. doi: 10.1359/JBMR.040405. [DOI] [PubMed] [Google Scholar]
- 84.Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 85.Moffett SP, Zmuda JM, Cauley JA, Stone KL, Nevitt MC, Ensrud KE, Hillier TA, Hochberg MC, Joslyn G, Morin P, Cummings SR. Association of the G-174C variant in the interleukin-6 promoter region with bone loss and fracture risk in older women. J Bone Miner Res. 2004;19:1612–1618. doi: 10.1359/JBMR.040707. [DOI] [PubMed] [Google Scholar]
- 86.Bollerslev J, Wilson SG, Dick IM, Devine A, Dhaliwal SS, Prince RL. Calcium-sensing receptor gene polymorphism A986S does not predict serum calcium level, bone mineral density, calcaneal ultrasound indices, or fracture rate in a large cohort of elderly women. Calcif Tissue Int. 2004;74:12–17. doi: 10.1007/s00223-002-0066-1. [DOI] [PubMed] [Google Scholar]
- 87.Dick IM, Devine A, Li S, Dhaliwal SS, Prince RL. The T869C TGF beta polymorphism is associated with fracture, bone mineral density, and calcaneal quantitative ultrasound in elderly women. Bone. 2003;33:335–341. doi: 10.1016/s8756-3282(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 88.Shearman AM, Karasik D, Gruenthal KM, Demissie S, Cupples LA, Housman DE, Kiel DP. Estrogen receptor beta polymorphisms are associated with bone mass in women and men: The Framingham Study. J Bone Miner Res. 2004;19:773–781. doi: 10.1359/JBMR.0301258. [DOI] [PubMed] [Google Scholar]
- 89.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 90.Yamada Y, Ando F, Niino N, Shimokata H. Association of polymorphisms of the osteoprotegerin gene with bone mineral density in Japanese women but not men. Mol Genet Metab. 2003;80:344–349. doi: 10.1016/S1096-7192(03)00125-2. [DOI] [PubMed] [Google Scholar]
- 91.Tofteng CL, Kindmark A, Brandstrom H, Abrahamsen B, Petersen S, Stiger F, Stilgren LS, Jensen JE, Vestergaard P, Langdahl BL, Mosekilde L. Polymorphisms in the CYP19 and AR genes–relation to bone mass and longitudinal bone changes in postmenopausal women with or without hormone replacement therapy: The Danish Osteoporosis Prevention Study. Calcif Tissue Int. 2004;74:25–34. doi: 10.1007/s00223-002-2158-3. [DOI] [PubMed] [Google Scholar]
- 92.Albagha OM, Tasker PN, McGuigan FE, Reid DM, Ralston SH. Linkage disequilibrium between polymorphisms in the human TNFRSF1B gene and their association with bone mass in perimenopausal women. Hum Mol Genet. 2002;11:2289–2295. doi: 10.1093/hmg/11.19.2289. [DOI] [PubMed] [Google Scholar]
- 93.Moffett SP, Zmuda JM, Oakley JI, Beck TJ, Cauley JA, Stone KL, Lui L, Ensrud KE, Hillier TA, Hochberg MC, Morin P, Green D, Peltz G. Tumor necrosis factor alpha polymorphism, bone strength phenotypes, and the risk of fracture in older women. J Bone Miner Res. 2004;19:S250. doi: 10.1210/jc.2004-2235. [DOI] [PubMed] [Google Scholar]
- 94.Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling: A union made for bone. J Bone Miner Res. 2004;19:1749–1757. doi: 10.1359/JBMR.040816. [DOI] [PubMed] [Google Scholar]
- 95.Johnson ML, Gong G, Kimberling W, Recker SM, Kimmel DB, Recker RB. Linkage of a gene causing high bone mass to human chromosome 11 (11q12–13) Am J Hum Genet. 1997;60:1326–1332. doi: 10.1086/515470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koh JM, Jung MH, Hong JS, Park HJ, Chang JS, Shin HD, Kim SY, Kim GS. Association between bone mineral density and LDL receptor-related protein 5 gene polymorphisms in young Korean men. J Korean Med Sci. 2004;19:407–412. doi: 10.3346/jkms.2004.19.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, Rizzoli R, Antonarakis SE. Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet. 2004;74:866–875. doi: 10.1086/420771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koller DL, Rodriguez LA, Christian JC, Slemenda CW, Econs MJ, Hui SL, Morin P, Conneally PM, Joslyn G, Curran ME, Peacock M, Johnston CC, Foroud T. Linkage of a QTL contributing to normal variation in bone mineral density to chromosome 11q12–13. J Bone Miner Res. 1998;13:1903–1908. doi: 10.1359/jbmr.1998.13.12.1903. [DOI] [PubMed] [Google Scholar]
- 100.Bollerslev J, Wilson SG, Dick IM, Islam AFM, Ueland T, Devine A, Prince RL. LRP5 gene polymorphisms predict bone mass and incident fractures in elderly Australian women. J Bone Miner Res. 2004;19:S383. doi: 10.1016/j.bone.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 101.Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerostenosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uitterlinden AG, Arp PP, Paeper BW, Charmley P, Proll S, Rivadeneira F, Fang Y, van Meurs JB, Britschgi TB, Latham JA, Schatzman RC, Pols HA, Brunkow ME. Polymorphisms in the sclerostenosis/van Buchem disease gene (SOST) region are associated with bone mineral density in elderly whites. Am J Hum Genet. 2004;75:1032–1045. doi: 10.1086/426458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Freimer N, Sabatti C. The use of pedigree, sib-pair and association studies of common diseases for genetic mapping and epidemiology. Nat Genet. 2004;36:1045–1051. doi: 10.1038/ng1433. [DOI] [PubMed] [Google Scholar]
- 104.Huizinga TW, Pisetsky DS, Kimberly RP. Associations, populations, and the truth: Recommendations for genetic association studies in arthritis and rheumatism. Arthritis Rheum. 2004;50:2066–2071. doi: 10.1002/art.20360. [DOI] [PubMed] [Google Scholar]
- 105.Cardon LR, Bell JI. Association study designs for complex diseases. Nat Rev Genet. 2001;2:91–99. doi: 10.1038/35052543. [DOI] [PubMed] [Google Scholar]
- 106.Romero R, Kuivaniemi H, Tromp G, Olson J. The design, execution, and interpretation of genetic association studies to decipher complex diseases. Am J Obstet Gynecol. 2002;187:1299–1312. doi: 10.1067/mob.2002.128319. [DOI] [PubMed] [Google Scholar]
- 107.Zondervan KT, Cardon LR. The complex interplay among factors that influence allelic association. Nat Rev Genet. 2004;5:89–100. doi: 10.1038/nrg1270. [DOI] [PubMed] [Google Scholar]
- 108.Ioannidis JP, Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG. Genetic associations in large versus small studies: An empirical assessment. Lancet. 2003;361:567–571. doi: 10.1016/S0140-6736(03)12516-0. [DOI] [PubMed] [Google Scholar]
- 109.Weiss KM, Clark AG. Linkage disequilibrium and the mapping of complex human traits. Trends Genet. 2002;18:19–24. doi: 10.1016/s0168-9525(01)02550-1. [DOI] [PubMed] [Google Scholar]
- 110.Ardlie KG, Kruglyak L, Seielstad M. Patterns of linkage disequilibrium in the human genome. Nat Rev Genet. 2002;3:299–309. doi: 10.1038/nrg777. [DOI] [PubMed] [Google Scholar]
- 111.Cardon LR, Abecasis GR. Using haplotype blocks to map human complex trait loci. Trends Genet. 2003;19:135–140. doi: 10.1016/S0168-9525(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 112.Shifman S, Kuypers J, Kokoris M, Yakir B, Darvasi A. Linkage disequilibrium patterns of the human genome across populations. Hum Mol Genet. 2003;12:771–776. doi: 10.1093/hmg/ddg088. [DOI] [PubMed] [Google Scholar]
- 113.Neale BM, Sham PC. The future of association studies: Gene-based analysis and replication. Am J Hum Genet. 2004;75:353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- 115.Doerge RW, Churchill GA. Permutation tests for multiple loci affecting a quantitative character. Genetics. 1996;142:285–294. doi: 10.1093/genetics/142.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McIntyre LM, Martin ER, Simonsen KL, Kaplan NL. Circumventing multiple testing: A multilocus Monte Carlo approach to testing for association. Genet Epidemiol. 2000;19:18–29. doi: 10.1002/1098-2272(200007)19:1<18::AID-GEPI2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 117.Deng HW. Population admixture may appear to mask, change or reverse genetic effects of genes underlying complex traits. Genetics. 2001;159:1319–1323. doi: 10.1093/genetics/159.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas DC, Witte JS. Point: Population stratification: A problem for case-control studies of candidate-gene associations? Cancer Epidemiol Biomarkers Prev. 2002;11:505–512. [PubMed] [Google Scholar]
- 119.Wacholder S, Rothman N, Caporaso N. Counterpoint: Bias from population stratification is not a major threat to the validity of conclusions from epidemiological studies of common polymorphisms and cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:513–520. [PubMed] [Google Scholar]
- 120.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- 121.Freedman ML, Reich D, Penney KL, McDonald GJ, Mignault AA, Patterson N, Gabriel SB, Topol EJ, Smoller JW, Pato CN, Pato MT, Petryshen TL, Kolonel LN, Lander ES, Sklar P, Henderson B, Hirschhorn JN, Altshuler D. Assessing the impact of population stratification on genetic association studies. Nat Genet. 2004;36:388–393. doi: 10.1038/ng1333. [DOI] [PubMed] [Google Scholar]
- 122.Marchini J, Cardon LR, Phillips MS, Donnelly P. The effects of human population structure on large genetic association studies. Nat Genet. 2004;36:512–517. doi: 10.1038/ng1337. [DOI] [PubMed] [Google Scholar]
- 123.Helgason A, Yngvadottir B, Hrafnkelsson B, Gulcher J, Stefansson K. An Icelandic example of the impact of population structure on association studies. Nat Genet. 2004;37:90–95. doi: 10.1038/ng1492. [DOI] [PubMed] [Google Scholar]
- 124.Spielman RS, Ewens WJ. The TDT and other family-based tests for linkage disequilibrium and association. Am J Hum Genet. 1996;59:983–989. [PMC free article] [PubMed] [Google Scholar]
- 125.Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: The insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am J Hum Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- 126.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 127.Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Orwoll ES, Belknap JK, Klein RF, Duncan EL, Cardon LR, Sinsheimer JS, Wass JA, Brown MA. Gender specificity in the genetic determinants of peak bone mass site and gender specificity of inheritance of bone mineral density. J Bone Miner Res. 2001;16:1962–1971. doi: 10.1359/jbmr.2001.16.11.1962. [DOI] [PubMed] [Google Scholar]
- 129.Duncan EL, Cardon LR, Sinsheimer JS, Wass JA, Brown MA. Site and gender specificity of inheritance of bone mineral density. J Bone Miner Res. 2003;18:1531–1538. doi: 10.1359/jbmr.2003.18.8.1531. [DOI] [PubMed] [Google Scholar]
- 130.Harris M, Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Genetic and environmental correlations between bone formation and bone mineral density: A twin study. J Bone Miner Res. 1998;22:141–145. doi: 10.1016/s8756-3282(97)00252-4. [DOI] [PubMed] [Google Scholar]
- 131.Deng HW, Mahaney MC, Williams JT, Li J, Conway T, Davies KM, Li JL, Deng H, Recker RR. Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet Epidemiol. 2002;22:12–25. doi: 10.1002/gepi.1040. [DOI] [PubMed] [Google Scholar]
- 132.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De PA, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van HW, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 133.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van EP, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heaney C, Shalev H, Elbedour K, Carmi R, Staack JB, Sheffield VC, Beier DR. Human autosomal recessive osteopetrosis maps to 11q13, a position predicted by comparative mapping of the murine osteosclerosis (oc) mutation. Hum Mol Genet. 1998;7:1407–1410. doi: 10.1093/hmg/7.9.1407. [DOI] [PubMed] [Google Scholar]
- 135.Deng HW, Xu FH, Conway T, Deng XT, Li JL, Davies KM, Deng H, Johnson M, Recker RR. Is population bone mineral density variation linked to the marker D11S987 on chromosome 11q12–13? J Clin Endocrinol Metab. 2001;86:3735–3741. doi: 10.1210/jcem.86.8.7762. [DOI] [PubMed] [Google Scholar]
- 136.Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 137.Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F, Recker RR. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35:162–169. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 138.Ezura Y, Urano T, Nakajima T, Sudo Y, Yoshida H, Suzuki T, Hosoi T, Inoue S, Shiraki M, Emi M. Association of single nucleotide polymorphisms in the low density lipoprotein receptor-related protein 5 gene (LRP5) with bone mineral density of adult women. J Bone Miner Res. 2004;19:S247. [Google Scholar]
- 139.Mizuguchi T, Furuta I, Watanabe Y, Tsukamoto K, Tomita H, Tsujihata M, Ohta T, Kishino T, Matsumoto N, Minakami H, Niikawa N, Yoshiura K. LRP5, low-density-lipoprotein-receptor-related protein 5, is a determinant for bone mineral density. J Hum Genet. 2004;49:80–86. doi: 10.1007/s10038-003-0111-6. [DOI] [PubMed] [Google Scholar]
- 140.Oakley JI, Moffett SP, Petro N, Cauley JA, Beck TJ, Bunker CH, Patrick AL, Wheeler VW, Ferrell RE, Zmuda JM. LRP5 amino acid substitutions: Differences in allele frequencies and association with bone density and structure in Afro-Caribbean men. J Bone Miner Res. 2004;19:S387. [Google Scholar]
- 141.Urano T, Shiraki M, Ezura Y, Fujita M, Sekine E, Hoshino S, Hosoi T, Orimo H, Emi M, Ouchi Y, Inoue S. Association of a single-nucleotide polymorphism in low-density lipoprotein receptor-related protein 5 gene with bone mineral density. J Bone Miner Metab. 2004;22:341–345. doi: 10.1007/s00774-003-0492-9. [DOI] [PubMed] [Google Scholar]
- 142.Econs MJ, Koller DL, Hui SL, Fishburn T, Conneally PM, Johnston CC, Jr, Peacock M, Foroud TM. Confirmation of linkage to chromosome 1q for peak vertebral bone mineral density in premenopausal white women. Am J Hum Genet. 2004;74:223–228. doi: 10.1086/381401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang QY, Xu FH, Shen H, Deng HY, Conway T, Liu YJ, Liu YZ, Li JL, Li MX, Davies KM, Recker RR, Deng HW. Genome scan for QTLs underlying bone size variation at 10 refined skeletal sites: Genetic heterogeneity and the significance of phenotype refinement. Physiol Genomics. 2004;17:326–331. doi: 10.1152/physiolgenomics.00161.2002. [DOI] [PubMed] [Google Scholar]
- 144.Kammerer CM, Schneider JL, Cole SA, Hixson JE, Samollow PB, O’Connell JR, Perez R, Dyer TD, Almasy L, Blangero J, Bauer RL, Mitchell BD. Quantitative trait loci on chromosomes 2p, 4p, and 13q influence bone mineral density of the forearm and hip in Mexican Americans. J Bone Miner Res. 2003;18:2245–2252. doi: 10.1359/jbmr.2003.18.12.2245. [DOI] [PubMed] [Google Scholar]
- 145.Karasik D, Cupples LA, Hannan MT, Kiel DP. Age, gender, and body mass effects on quantitative trait loci for bone mineral density: The Framingham Study. Bone. 2003;33:308–316. doi: 10.1016/s8756-3282(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 146.Karasik D, Cupples LA, Hannan MT, Kiel DP. Genome screen for a combined bone phenotype using principal component analysis: The Framingham study. Bone. 2004;34:547–556. doi: 10.1016/j.bone.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 147.Koller DL, White KE, Liu G, Hui SL, Conneally PM, Johnston CC, Econs MJ, Foroud T, Peacock M. Linkage of structure at the proximal femur to chromosomes 3, 7, 8, and 19. J Bone Miner Res. 2003;18:1057–1065. doi: 10.1359/jbmr.2003.18.6.1057. [DOI] [PubMed] [Google Scholar]
- 148.Peacock M, Koller DL, Hui S, Johnston CC, Foroud T, Econs MJ. Peak bone mineral density at the hip is linked to chromosomes 14q and 15q. Osteoporos Int. 2004;15:489–496. doi: 10.1007/s00198-003-1560-7. [DOI] [PubMed] [Google Scholar]
- 149.Shen H, Zhang YY, Long JR, Xu FH, Liu YZ, Xiao P, Zhao LJ, Xiong DH, Liu YJ, Dvornyk V, Rocha-Sanchez S, Liu PY, Li JL, Conway T, Davies KM, Recker RR, Deng HW. A genome-wide linkage scan for bone mineral density in an extended sample: Evidence for linkage on 11q23 and Xq27. J Med Genet. 2004;41:743–751. doi: 10.1136/jmg.2004.020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD, Sigurdardottir MS, Bagger Y, Christiansen C, Reynisdottir I, Grant SF, Jonasson K, Frigge ML, Gulcher JR, Sigurdsson G, Stefansson K. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 2003;1:E69. doi: 10.1371/journal.pbio.0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wilson SG, Reed PW, Andrew T, Barber MJ, Lindersson M, Langdown M, Thompson D, Thompson E, Bailey M, Chiano M, Kleyn PW, Spector TD. A genome-screen of a large twin cohort reveals linkage for quantitative ultrasound of the calcaneus to 2q33–37 and 4q12–21. J Bone Miner Res. 2004;19:270–277. doi: 10.1359/JBMR.0301224. [DOI] [PubMed] [Google Scholar]
- 152.Huang QY, Xu FH, Shen H, Zhao LJ, Deng HY, Liu YJ, Dvomyk V, Conway T, Davies KM, Li JL, Liu YZ, Recker RR, Deng HW. A second-stage genome scan for QTLs influencing BMD variation. Calcif Tissue Int. 2004;75:138–143. doi: 10.1007/s00223-004-0088-y. [DOI] [PubMed] [Google Scholar]
- 153.Xu FH, Liu YJ, Deng H, Huang QY, Zhao LJ, Shen H, Liu YZ, Dvornyk V, Conway T, Li JL, Davies KM, Recker RR, Deng HW. A follow-up linkage study for bone size variation in an extended sample. Bone. 2004;35:777–784. doi: 10.1016/j.bone.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 154.Deng HW, Shen H, Xu FH, Deng H, Conway T, Liu YJ, Liu YZ, Li JL, Huang QY, Davies KM, Recker RR. Several genomic regions potentially containing QTLs for bone size variation were identified in a whole-genome linkage scan. Am J Med Genet A. 2003;119:121–131. doi: 10.1002/ajmg.a.20100. [DOI] [PubMed] [Google Scholar]
- 155.Deng HW, Xu FH, Huang QY, Shen H, Deng H, Conway T, Liu YJ, Liu YZ, Li JL, Zhang HT, Davies KM, Recker RR. A whole-genome linkage scan suggests several genomic regions potentially containing quantitative trait Loci for osteoporosis. J Clin Endocrinol Metab. 2002;87:5151–5159. doi: 10.1210/jc.2002-020474. [DOI] [PubMed] [Google Scholar]
- 156.Karasik D, Myers RH, Cupples LA, Hannan MT, Gagnon DR, Herbert A, Kiel DP. Genome screen for quantitative trait loci contributing to normal variation in bone mineral density: The Framingham Study. J Bone Miner Res. 2002;17:1718–1727. doi: 10.1359/jbmr.2002.17.9.1718. [DOI] [PubMed] [Google Scholar]
- 157.Koller DL, Liu G, Econs MJ, Hui SL, Morin PA, Joslyn G, Rodriguez LA, Conneally PM, Christian JC, Johnston CC, Jr, Foroud T, Peacock M. Genome screen for quantitative trait loci underlying normal variation in femoral structure. J Bone Miner Res. 2001;16:985–991. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- 158.Koller DL, Econs MJ, Morin PA, Christian JC, Hui SL, Parry P, Curran ME, Rodriguez LA, Conneally PM, Joslyn G, Peacock M, Johnston CC, Foroud T. Genome screen for QTLs contributing to normal variation in bone mineral density and osteoporosis. J Clin Endocrinol Metab. 2000;85:3116–3120. doi: 10.1210/jcem.85.9.6778. [DOI] [PubMed] [Google Scholar]
- 159.Devoto M, Shimoya K, Caminis J, Ott J, Tenenhouse A, Whyte MP, Sereda L, Hall S, Considine E, Williams CJ, Tromp G, Kuivaniemi H, la-Kokko L, Prockop DJ, Spotila LD. First-stage autosomal genome screen in extended pedigrees suggests genes predisposing to low bone mineral density on chromosomes 1p, 2p and 4q. Eur J Hum Genet. 1998;6:151–157. doi: 10.1038/sj.ejhg.5200169. [DOI] [PubMed] [Google Scholar]
- 160.Klein RF, Mitchell SR, Phillips TJ, Belknap JK, Orwoll ES. Quantitative trait loci affecting peak bone mineral density in mice. J Bone Miner Res. 1998;13:1648–1656. doi: 10.1359/jbmr.1998.13.11.1648. [DOI] [PubMed] [Google Scholar]
- 161.Wilson SG, Reed PW, Andrew T, Langdown M, Prince RL, Spector TD. Fine mapping provides further evidence of linkage for bone mineral density to 3p21. J Bone Miner Res. 2004;19:S154. [Google Scholar]
- 162.Wilson SG, Reed PW, Bansal A, Chiano M, Lindersson M, Langdown M, Prince RL, Thompson D, Thompson E, Bailey M, Kleyn PW, Sambrook P, Shi MM, Spector TD. Comparison of genome screens for two independent cohorts provides replication of suggestive linkage of bone mineral density to 3p21 and 1p36. Am J Hum Genet. 2003;72:144–155. doi: 10.1086/345819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koller DL, Hui SL, Johnston CC, Econs MJ, Foroud T, Peacock M. Genome screen for femoral structure QTLs in men. J Bone Miner Res. 2004;19:S156. doi: 10.1359/jbmr.2001.16.6.985. [DOI] [PubMed] [Google Scholar]
- 164.Czerwinski SA, Lee M, Choh AC, Demerath EW, Chumlea WC, Sun SS, Siervogel RM, Towne B. Genome-wide scan for QTL underlying normal variation in calcaneal quantitative ultrasound measures: The Fels Longitudinal Study. J Bone Miner Res. 2004;19:S157. [Google Scholar]
- 165.Shimizu M, Higuchi K, Bennett B, Xia C, Tsuboyama T, Kasai S, Chiba T, Fujisawa H, Kogishi K, Kitado H, Kimoto M, Takeda N, Matsushita M, Okumura H, Serikawa T, Nakamura T, Johnson TE, Hosokawa M. Identification of peak bone mass QTL in a spontaneously osteoporotic mouse strain. Mamm Genome. 1999;10:81–87. doi: 10.1007/s003359900949. [DOI] [PubMed] [Google Scholar]
- 166.Benes H, Weinstein RS, Zheng W, Thaden JJ, Jilka RL, Manolagas SC, Shmookler Reis RJ. Chromosomal mapping of osteopenia-associated quantitative trait loci using closely related mouse strains. J Bone Miner Res. 2000;15:626–633. doi: 10.1359/jbmr.2000.15.4.626. [DOI] [PubMed] [Google Scholar]
- 167.Klein OF, Carlos AS, Vartanian KA, Chambers VK, Turner EJ, Phillips TJ, Belknap JK, Orwoll ES. Confirmation and fine mapping of chromosomal regions influencing peak bone mass in mice. J Bone Miner Res. 2001;16:1953–1961. doi: 10.1359/jbmr.2001.16.11.1953. [DOI] [PubMed] [Google Scholar]
- 168.Beamer WG, Shultz KL, Churchill GA, Frankel WN, Baylink DJ, Rosen CJ, Donahue LR. Quantitative trait loci for bone density in C57BL/6J and CAST/EiJ inbred mice. Mamm Genome. 1999;10:1043–1049. doi: 10.1007/s003359901159. [DOI] [PubMed] [Google Scholar]
- 169.Beamer WG, Shultz KL, Donahue LR, Churchill GA, Sen S, Wergedal JR, Baylink DJ, Rosen CJ. Quantitative trait loci for femoral and lumbar vertebral bone mineral density in C57BL/6J and C3H/HeJ inbred strains of mice. J Bone Miner Res. 2001;16:1195–1206. doi: 10.1359/jbmr.2001.16.7.1195. [DOI] [PubMed] [Google Scholar]
- 170.Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science. 2004;303:229–232. doi: 10.1126/science.1090985. [DOI] [PubMed] [Google Scholar]
- 171.Shultz KL, Donahue LR, Bouxsein ML, Baylink DJ, Rosen CJ, Beamer WG. Congenic strains of mice for verification and genetic decomposition of quantitative trait loci for femoral bone mineral density. J Bone Miner Res. 2003;18:175–185. doi: 10.1359/jbmr.2003.18.2.175. [DOI] [PubMed] [Google Scholar]
- 172.Turner CH, Sun Q, Schriefer J, Pitner N, Price R, Bouxsein ML, Rosen CJ, Donahue LR, Shultz KL, Beamer WG. Congenic mice reveal sex-specific genetic regulation of femoral structure and strength. Calcif Tissue Int. 2003;73:297–303. doi: 10.1007/s00223-002-1062-1. [DOI] [PubMed] [Google Scholar]
- 173.Koller DL, Schriefer J, Sun Q, Shultz KL, Donahue LR, Rosen CJ, Foroud T, Beamer WG, Turner CH. Genetic effects for femoral biomechanics, structure, and density in C57BL/6J and C3H/HeJ inbred mouse strains. J Bone Miner Res. 2003;18:1758–1765. doi: 10.1359/jbmr.2003.18.10.1758. [DOI] [PubMed] [Google Scholar]
- 174.Volkman SK, Galecki AT, Burke DT, Paczas MR, Moalli MR, Miller RA, Goldstein SA. Quantitative trait loci for femoral size and shape in a genetically heterogeneous mouse population. J Bone Miner Res. 2003;18:1497–1505. doi: 10.1359/jbmr.2003.18.8.1497. [DOI] [PubMed] [Google Scholar]
- 175.Volkman SK, Galecki AT, Burke DT, Miller RA, Goldstein SA. Quantitative trait loci that modulate femoral mechanical properties in a genetically heterogeneous mouse population. J Bone Miner Res. 2004;19:1497–1505. doi: 10.1359/JBMR.040506. [DOI] [PubMed] [Google Scholar]
- 176.Bouxsein ML, Uchiyama T, Rosen CJ, Shultz KL, Donahue LR, Turner CH, Sen S, Churchill GA, Muller R, Beamer WG. Mapping quantitative trait loci for vertebral trabecular bone volume fraction and microarchitecture in mice. J Bone Miner Res. 2004;19:587–599. doi: 10.1359/JBMR.0301255. [DOI] [PubMed] [Google Scholar]
- 177.Masinde GL, Wergedal J, Davidson H, Mohan S, Li R, Li X, Baylink DJ. Quantitative trait loci for periosteal circumference (PC): Identification of single loci and epistatic effects in F2 MRL/SJL mice. Bone. 2003;32:554–560. doi: 10.1016/s8756-3282(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 178.Robling AG, Li J, Shultz KL, Beamer WG, Turner CH. Evidence for a skeletal mechanosensitivity gene on mouse chromosome 4. FASEB J. 2003;17:324–326. doi: 10.1096/fj.02-0393fje. [DOI] [PubMed] [Google Scholar]
- 179.Srivastava AK, Masinde G, Yu H, Baylink DJ, Mohan S. Mapping quantitative trait loci that influence blood levels of alkaline phosphatase in MRL/MpJ and SJL/J mice. Bone. 2004;35:1086–1094. doi: 10.1016/j.bone.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 180.Mohan S, Masinde G, Li X, Baylink DJ. Mapping quantitative trait loci that influence serum insulin-like growth factor binding protein-5 levels in F2 mice (MRL/MpJ X SJL/J) Endocrinology. 2003;144:3491–3496. doi: 10.1210/en.2003-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lockhart DJ, Winzeler EA. Genomics, gene expression and DNA arrays. Nature. 2000;405:827–836. doi: 10.1038/35015701. [DOI] [PubMed] [Google Scholar]
- 182.Heller RA, Schena M, Chai A, Shalon D, Bedilion T, Gilmore J, Woolley DE, Davis RW. Discovery and analysis of inflammatory disease-related genes using cDNA microarrays. Proc Natl Acad Sci USA. 1997;94:2150–2155. doi: 10.1073/pnas.94.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chiba S, Un-No M, Neer RM, Okada K, Serge GV, Lee K. Parathyroid hormone induces interleukin-6 gene expression in bone stromal cells of young rats. J Vet Med Sci. 2002;64:641–644. doi: 10.1292/jvms.64.641. [DOI] [PubMed] [Google Scholar]
- 184.Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- 185.Hughes TR, Marton MJ, Jones AR, Roberts CJ, Stoughton R, Armour CD, Bennett HA, Coffey E, Dai H, He YD, Kidd MJ, King AM, Meyer MR, Slade D, Lum PY, Stepaniants SB, Shoemaker DD, Gachotte D, Chakraburtty K, Simon J, Bard M, Friend SH. Functional discovery via a compendium of expression profiles. Cell. 2000;102:109–126. doi: 10.1016/s0092-8674(00)00015-5. [DOI] [PubMed] [Google Scholar]
- 186.Liu F, Aubin JE, Malaval L. Expression of leukemia inhibitory factor (LIF)/interleukin-6 family cytokines and receptors during in vitro osteogenesis: Differential regulation by dexamethasone and LIF. Bone. 2002;31:212–219. doi: 10.1016/s8756-3282(02)00806-2. [DOI] [PubMed] [Google Scholar]
- 187.Power RA, Iwaniec UT, Wronski TJ. Changes in gene expression associated with the bone anabolic effects of basic fibroblast growth factor in aged ovariectomized rats. Bone. 2002;31:143–148. doi: 10.1016/s8756-3282(02)00799-8. [DOI] [PubMed] [Google Scholar]
- 188.Srivastava S, Weitzmann MN, Kimble RB, Rizzo M, Zahner M, Milbrandt J, Ross FP, Pacifici R. Estrogen blocks M-CSF gene expression and osteoclast formation by regulating phosphorylation of Egr-1 and its interaction with Sp-1. J Clin Invest. 1998;102:1850–1859. doi: 10.1172/JCI4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tomlinson JW, Bujalska I, Stewart PM, Cooper MS. The role of 11 beta-hydroxysteroid dehydrogenase in central obesity and osteoporosis. Endocr Res. 2000;26:711–722. doi: 10.3109/07435800009048591. [DOI] [PubMed] [Google Scholar]
- 190.Locklin RM, Riggs BL, Hicok KC, Horton HF, Byrne MC, Khosla S. Assessment of gene regulation by bone morphogenetic protein 2 in human marrow stromal cells using gene array technology. J Bone Miner Res. 2001;16:2192–2204. doi: 10.1359/jbmr.2001.16.12.2192. [DOI] [PubMed] [Google Scholar]
- 191.Chen J, Zhong Q, Wang J, Cameron RS, Borke JL, Isales CM, Bollag RJ. Microarray analysis of Tbx2-directed gene expression: A possible role in osteogenesis. Mol Cell Endocrinol. 2001;177:43–54. doi: 10.1016/s0303-7207(01)00456-7. [DOI] [PubMed] [Google Scholar]
- 192.Beck GR, Jr, Zerler B, Moran E. Gene array analysis of osteoblast differentiation. Cell Growth Differ. 2001;12:61–83. [PubMed] [Google Scholar]
- 193.Connor JR, Kumar S, Sathe G, Mooney J, O’Brien SP, Mui P, Murdock PR, Gowen M, Lark MW. Clusterin expression in adult human normal and osteoarthritic articular cartilage. Osteoarthritis Cartilage. 2001;9:727–737. doi: 10.1053/joca.2001.0475. [DOI] [PubMed] [Google Scholar]
- 194.Stokes DG, Liu G, Coimbra IB, Piera-Velazquez S, Crowl RM, Jimenez SA. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 2002;46:404–419. doi: 10.1002/art.10106. [DOI] [PubMed] [Google Scholar]
- 195.Vincenti MP, Brinckerhoff CE. Early response genes induced in chondrocytes stimulated with the inflammatory cytokine interleukin-1beta. Arthritis Res. 2001;3:381–388. doi: 10.1186/ar331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Furushima K, Shimo-Onoda K, Maeda S, Nobukuni T, Ikari K, Koga H, Komiya S, Nakajima T, Harata S, Inoue I. Large-scale screening for candidate genes of ossification of the posterior longitudinal ligament of the spine. J Bone Miner Res. 2002;17:128–137. doi: 10.1359/jbmr.2002.17.1.128. [DOI] [PubMed] [Google Scholar]
- 197.Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C, Trepel J, Meltzer P, Helman L. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res. 2001;61:3750–3759. [PubMed] [Google Scholar]
- 198.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532–539. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 199.Theilhaber J, Connolly T, Roman-Roman S, Bushnell S, Jackson A, Call K, Garcia T, Baron R. Finding genes in the C2C12 osteogenic pathway by k-nearest-neighbor classification of expression data. Genome Res. 2002;12:165–176. doi: 10.1101/gr.182601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gu W, Li X, Lau KH, Edderkaoui B, Donahae LR, Rosen CJ, Beamer WG, Shultz KL, Srivastava A, Mohan S, Baylink DJ. Gene expression between a congenic strain that contains a quantitative trait locus of high bone density from CAST/EiJ and its wild-type strain C57BL/6J. Funct Integr Genomics. 2002;1:375–386. doi: 10.1007/s10142-001-0042-2. [DOI] [PubMed] [Google Scholar]
- 201.Doi M, Nagano A, Nakamura Y. Genome-wide screening by cDNA microarray of genes associated with matrix mineralization by human mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2002;290:381–390. doi: 10.1006/bbrc.2001.6196. [DOI] [PubMed] [Google Scholar]
- 202.Raouf A, Seth A. Discovery of osteoblast-associated genes using cDNA microarrays. Bone. 2002;30:463–471. doi: 10.1016/s8756-3282(01)00699-8. [DOI] [PubMed] [Google Scholar]
- 203.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 204.Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: Comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 205.Takahashi N, Udagawa NT, Takami M. Cells of bone: Osteoclast generation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. Academic Press; San Diego, CA, USA: 2002. pp. 109–126. [Google Scholar]
- 206.Liu YZ, Dvornyk V, Liu YJ, Shen H, Chang J, Recker R, Deng HW. Microarray study of circulating monocytes in search for functional genes for osteoporosis. J Bone Miner Res. 2004;19:S150. [Google Scholar]
- 207.Kamme F, Erlander MG. Global gene expression analysis of single cells. Curr Opin Drug Discov Devel. 2003;6:231–236. [PubMed] [Google Scholar]
- 208.Kamme F, Salunga R, Yu J, Tran DT, Zhu J, Luo L, Bittner A, Guo HQ, Miller N, Wan J, Erlander M. Single-cell microarray analysis in hippocampus CA1: Demonstration and validation of cellular heterogeneity. J Neurosci. 2003;23:3607–3615. doi: 10.1523/JNEUROSCI.23-09-03607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sanz E, Alvarez-Mon M, Martinez-A C, de la Hera A. Human cord blood CD34+Pax-5+ B-cell progenitors: Single-cell analyses of their gene expression profiles. Blood. 2003;101:3424–3430. doi: 10.1182/blood-2002-07-2244. [DOI] [PubMed] [Google Scholar]
- 210.Abbott A. A post-genomic challenge: Learning to read patterns of protein synthesis. Nature. 1999;402:715–720. doi: 10.1038/45350. [DOI] [PubMed] [Google Scholar]
- 211.Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- 212.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK, Bumgarner R, Goodlett DR, Aebersold R, Hood L. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science. 2001;292:929–934. doi: 10.1126/science.292.5518.929. [DOI] [PubMed] [Google Scholar]
- 214.Banks RE, Dunn MJ, Hochstrasser DF, Sanchez JC, Blackstock W, Pappin DJ, Selby PJ. Proteomics: New perspectives, new biomedical opportunities. Lancet. 2000;356:1749–1756. doi: 10.1016/S0140-6736(00)03214-1. [DOI] [PubMed] [Google Scholar]
- 215.Graves PR, Haystead TA. Molecular biologist’s guide to proteomics. Microbiol Mol Biol Rev. 2002;66:39–63. doi: 10.1128/MMBR.66.1.39-63.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Blackstock WP, Weir MP. Proteomics: Quantitative and physical mapping of cellular proteins. Trends Biotechnol. 1999;17:121–127. doi: 10.1016/s0167-7799(98)01245-1. [DOI] [PubMed] [Google Scholar]
- 217.Evans CA, Tonge R, Blinco D, Pierce A, Shaw J, Lu Y, Hamzah HG, Gray A, Downes CP, Gaskell SJ, Spooncer E, Whetton AD. Comparative proteomics of primitive hematopoietic cell populations reveals differences in expression of proteins regulating motility. Blood. 2004;103:3751–3759. doi: 10.1182/blood-2003-09-3294. [DOI] [PubMed] [Google Scholar]
- 218.Brown RE, Boyle JL. Mesenchymal chondrosarcoma: Molecular characterization by a proteomic approach, with morphogenic and therapeutic implications. Ann Clin Lab Sci. 2003;33:131–141. [PubMed] [Google Scholar]
- 219.Behnam K, Murray SS, Whitelegge JP, Brochmann EJ. Identification of the molecular chaperone alpha B-crystallin in demineralized bone powder and osteoblast-like cells. J Orthop Res. 2002;20:1190–1196. doi: 10.1016/S0736-0266(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 220.Kubota K, Wakabayashi K, Matsuoka T. Proteome analysis of secreted proteins during osteoclast differentiation using two different methods: Two-dimensional electrophoresis and isotope-coded affinity tags analysis with two-dimensional chromatography. Proteomics. 2003;3:616–626. doi: 10.1002/pmic.200300410. [DOI] [PubMed] [Google Scholar]
- 221.Nuttall ME. Drug discovery and target validation. Cells Tissues Organs. 2001;169:265–271. doi: 10.1159/000047890. [DOI] [PubMed] [Google Scholar]
- 222.Lorenz P, Ruschpler P, Koczan D, Stiehl P, Thiesen HJ. From transcriptome to proteome: Differentially expressed proteins identified in synovial tissue of patients suffering from rheumatoid arthritis and osteoarthritis by an initial screen with a panel of 791 antibodies. Proteomics. 2003;3:991–1002. doi: 10.1002/pmic.200300412. [DOI] [PubMed] [Google Scholar]
- 223.Liu XH, Liu YJ, Jiang DK, Li YM, Li MX, Qin YJ, Jian WX, Zhou Q, Deng HW. No evidence for linkage and/or association of human alpha2-HS glycoprotein gene with bone mineral density variation in Chinese nuclear families. Calcif Tissue Int. 2003;73:244–250. doi: 10.1007/s00223-002-0005-1. [DOI] [PubMed] [Google Scholar]
- 224.Schoofs MW, van der KM, Hofman A, van Duijn CM, Stricker BH, Pols HA, Uitterlinden AG. ApoE gene polymorphisms, BMD, and fracture risk in elderly men and women: The Rotterdam study. J Bone Miner Res. 2004;19:1490–1496. doi: 10.1359/JBMR.040605. [DOI] [PubMed] [Google Scholar]
- 225.Efstathiadou Z, Koukoulis G, Stakias N, Challa A, Tsatsoulis A. Apolipoprotein E polymorphism is not associated with spinal bone mineral density in peri- and postmenopausal Greek women. Maturitas. 2004;48:259–264. doi: 10.1016/j.maturitas.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 226.Salmen T, Heikkinen AM, Mahonen A, Kroger H, Komulainen M, Pallonen H, Saarikoski S, Honkanen R, Maenpaa PH. Relation of androgen receptor gene polymorphism to bone mineral density and fracture risk in early postmenopausal women during a 5-year randomized hormone replacement therapy trial. J Bone Miner Res. 2003;18:319–324. doi: 10.1359/jbmr.2003.18.2.319. [DOI] [PubMed] [Google Scholar]
- 227.Langdahl BL, Stenkjaer L, Carstens M, Tofteng CL, Eriksen EF. A CAG repeat polymorphism in the androgen receptor gene is associated with reduced bone mass and increased risk of osteoporotic fractures. Calcif Tissue Int. 2003;73:237–243. doi: 10.1007/s00223-002-0019-8. [DOI] [PubMed] [Google Scholar]
- 228.Chen HY, Chen WC, Wu MC, Tsai FJ, Tsai CH. Androgen receptor (AR) gene microsatellite polymorphism in postmenopausal women: Correlation to bone mineral density and susceptibility to osteoporosis. Eur J Obstet Gynecol Reprod Biol. 2003;107:52–56. doi: 10.1016/s0301-2115(02)00315-9. [DOI] [PubMed] [Google Scholar]
- 229.Ebeling PR, Hussein Z, Ellis J, Lamantia A, Greenland K, Yeung S, Wong Z, Zajac JD, Harrap S. Androgen receptor CAG repeat polymorphism and bone density: A family and case-control association study in men with primary osteoporosis. J Bone Miner Res. 2004;19:S380. [Google Scholar]
- 230.Kenny AM, McGee D, Joseph C, Abreu C, Raisz LG. Lack of association between androgen receptor polymorphisms and bone mineral density or physical function in older men. J Bone Miner Res. 2004;19:S381. doi: 10.1080/07435800500406221. [DOI] [PubMed] [Google Scholar]
- 231.Mo XY, Cao CK, Xu FH, Liu MY, Li MX, Qin YJ, Zhou Q, Zhang YY, Deng HW. Lack of association between the HindIII RFLP of the osteocalcin (BGP) gene and bone mineral density (BMD) in healthy pre- and postmenopausal Chinese women. J Bone Miner Metab. 2004;22:264–269. doi: 10.1007/s00774-003-0478-7. [DOI] [PubMed] [Google Scholar]
- 232.Ichikawa S, Koller DL, Johnson ML, Lai D, Johnston CC, Hui SL, Foroud TM, Peacock M, Econs MJ. Polymorphisms in the bone morphogenetic protein 2 (BMP2) gene do not affect peak bone mineral density in men and women. J Bone Miner Res. 2004;19:S249. doi: 10.1007/s00198-005-0018-5. [DOI] [PubMed] [Google Scholar]
- 233.Cetani F, Pardi E, Borsari S, Vignali E, Dipollina G, Braga V, Adami S, Pinchera A, Marcocci C. Calcium-sensing receptor gene polymorphism is not associated with bone mineral density in Italian postmenopausal women. Eur J Endocrinol. 2003;148:603–607. doi: 10.1530/eje.0.1480603. [DOI] [PubMed] [Google Scholar]
- 234.Young R, Wu F, Van de Water N, Ames R, Gamble G, Reid IR. Calcium sensing receptor gene A986S polymorphism and responsiveness to calcium supplementation in postmenopausal women. J Clin Endocrinol Metab. 2003;88:697–700. doi: 10.1210/jc.2002-020355. [DOI] [PubMed] [Google Scholar]
- 235.Takacs I, Speer G, Bajnok E, Tabak A, Nagy Z, Horvath C, Kovacs K, Lakatos P. Lack of association between calcium-sensing receptor gene “A986S” polymorphism and bone mineral density in Hungarian postmenopausal women. Bone. 2002;30:849–852. doi: 10.1016/s8756-3282(02)00741-x. [DOI] [PubMed] [Google Scholar]
- 236.Bajnok E, Lakatos G, Tabak A, Takacs I, Horvath C, Kosa J, Speer G, Madarasz E, Nagy Z, Lakatos P. Association between collagen type I alpha1 (COL1A1) gene Sp1-, calcium-sensing receptor (CaSR) gene A986S-, interleukin-1 receptor antagonist (IL-1LN) gene VNTR polymorphisms and osteoporosis; their impact in prediction of osteoporotic bone fracture. J Bone Miner Res. 2004;19:S384. [Google Scholar]
- 237.Kim J, Ku S, Suh C, Kim S, Choi Y, Moon S. Relationship among calcium sensing receptor gene (CA) polymorphism, bone mineral density and bone responsiveness to hormone replacement therapy in postmenopausal Korean women. J Bone Miner Res. 2004;19:S245. [Google Scholar]
- 238.Balcells S, Bustamante M, Enjuanes A, Garcia-Giralt N, Aymar I, Nogu X, Mellibovsky L, Grinberg D. COL1A1, but not ESR1 or VDR polymorphisms are associated with BMD in a cohort of Spanish postmenopausal women. J Bone Miner Res. 2004;19:S249. [Google Scholar]
- 239.Willing MC, Torner JC, Burns TL, Janz KF, Marshall T, Gilmore J, Deschenes SP, Warren JJ, Levy SM. Gene polymorphisms, bone mineral density and bone mineral content in young children: The Iowa Bone Development Study. Osteoporos Int. 2003;14:650–658. doi: 10.1007/s00198-003-1416-1. [DOI] [PubMed] [Google Scholar]
- 240.van der Sluis IM, de Muinck Keizer-Schrama SM, Krenning EP, Pols HA, Uitterlinden AG. Vitamin D receptor gene polymorphism predicts height and bone size, rather than bone density in children and young adults. Calcif Tissue Int. 2003;73:332–338. doi: 10.1007/s00223-002-2130-2. [DOI] [PubMed] [Google Scholar]
- 241.Sapir-Koren R, Livshits G, Kobyliansky E. Association and linkage disequilibrium analyses suggest genetic effects of estrogen receptor alpha and collagen IA1 genes on bone mineral density in Caucasian women. Calcif Tissue Int. 2003;72:643–650. doi: 10.1007/s00223-002-2006-5. [DOI] [PubMed] [Google Scholar]
- 242.Pluijm SM, van Essen HW, Bravenboer N, Uitterlinden AG, Smit JH, Pols HA, Lips P. Collagen type I alpha1 Sp1 polymorphism, osteoporosis, and intervertebral disc degeneration in older men and women. Ann Rheum Dis. 2004;63:71–77. doi: 10.1136/ard.2002.002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Alvarez-Hernandez D, Naves M, Diaz-Lopez JB, Gomez C, Santamaria I, Cannata-Andia JB. Influence of polymorphisms in VDR and COLIA1 genes on the risk of osteoporotic fractures in aged men. Kidney Int. 2003;85:S14–S18. doi: 10.1046/j.1523-1755.63.s85.5.x. [DOI] [PubMed] [Google Scholar]
- 244.van der Sluis IM, de Muinck Keizer-Schrama SM, Pols HA, Lequin MH, Krenning EP, Uitterlinden AG. Collagen Ialpha1 polymorphism is associated with bone characteristics in Caucasian children and young adults. Calcif Tissue Int. 2002;71:393–399. doi: 10.1007/s00223-001-2093-8. [DOI] [PubMed] [Google Scholar]
- 245.Barros ER, Kasamatsu TS, Ramalho AC, Hauache OM, Vieira JG, Lazaretti-Castro M. Bone mineral density in young women of the city of Sao Paulo, Brazil: Correlation with both collagen type I alpha 1 gene polymorphism and clinical aspects. Braz J Med Biol Res. 2002;35:885–893. doi: 10.1590/s0100-879x2002000800005. [DOI] [PubMed] [Google Scholar]
- 246.Kann P, Bergink AP, Fang Y, Van Daele PL, Hofman A, van Leeuwen JP, Beyer J, Uitterlinden AG, Pols HA. The collagen Ia1 SP1 polymorphism is associated with differences in ultrasound transmission velocity in the calcaneus in postmenopausal women. Calcif Tissue Int. 2002;70:450–456. doi: 10.1007/s002230020007. [DOI] [PubMed] [Google Scholar]
- 247.Liu PY, Lu Y, Long JR, Xu FH, Shen H, Recker RR, Deng HW. Common variants at the PCOL2 and Sp1 binding sites of the COL1A1 gene and their interactive effect influence bone mineral density in Caucasians. J Med Genet. 2004;41:752–757. doi: 10.1136/jmg.2004.019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Long JR, Liu PY, Lu Y, Dvornyk V, Xiong DH, Zhao LJ, Deng HW. Tests of linkage and/or association of TGF-beta1 and COL1A1 genes with bone mass. Osteoporos Int. 2005;16:86–92. doi: 10.1007/s00198-004-1650-1. [DOI] [PubMed] [Google Scholar]
- 249.Lau EM, Choy DT, Li M, Woo J, Chung T, Sham A. The relationship between COLI A1 polymorphisms (Sp 1) and COLI A2 polymorphisms (Eco R1 and Puv II) with bone mineral density in Chinese men and women. Calcif Tissue Int. 2004;75:133–137. doi: 10.1007/s00223-003-0008-6. [DOI] [PubMed] [Google Scholar]
- 250.Deng FY, Liu MY, Li MX, Lei SF, Qin YJ, Zhou Q, Liu YJ, Deng HW. Tests of linkage and association of the COL1A2 gene with bone phenotypes’ variation in Chinese nuclear families. Bone. 2003;33:614–619. doi: 10.1016/s8756-3282(03)00234-5. [DOI] [PubMed] [Google Scholar]
- 251.Suuriniemi M, Mahonen A, Lyytikainen A, Kovanen V, Alen M, Cheng S. Faster bone growth during puberty contributed by the COL1A2 polymorphism: A benefit or risk? J Bone Miner Res. 2004;19:S383. [Google Scholar]
- 252.Tofteng CL, Abrahamsen B, Jensen JE, Petersen S, Teilmann J, Kindmark A, Vestergaard P, Gram J, Langdahl BL, Mosekilde L. Two single nucleotide polymorphisms in the CYP17 and COMT Genes–relation to bone mass and longitudinal bone changes in postmenopausal women with or without hormone replacement therapy. The Danish Osteoporosis Prevention Study. Calcif Tissue Int. 2004;75:123–132. doi: 10.1007/s00223-004-0176-z. [DOI] [PubMed] [Google Scholar]
- 253.Lorentzon M, Eriksson AL, Mellstrom D, Ohlsson C. The COMT val158met polymorphism is associated with peak BMD in men. J Bone Miner Res. 2004;19:2005–2011. doi: 10.1359/JBMR.040909. [DOI] [PubMed] [Google Scholar]
- 254.Zofkova I, Zajickova K, Hill M, Krepelova A. Does polymorphism C1377T of the calcitonin receptor gene determine bone mineral density in postmenopausal women? Exp Clin Endocrinol Diabetes. 2003;111:447–449. doi: 10.1055/s-2003-44293. [DOI] [PubMed] [Google Scholar]
- 255.Kim JG, Choi YM, Moon SY, Lee JY. Association of the calcitonin gene (CA) polymorphism with bone mass and bone responsiveness to hormone therapy in postmenopausal Korean women. Menopause. 2003;10:544–549. doi: 10.1097/01.GME.0000070525.74726.51. [DOI] [PubMed] [Google Scholar]
- 256.Tsai FJ, Chen WC, Chen HY, Tsai CH. The ALUI calcitonin receptor gene polymorphism (TT) is associated with low bone mineral density and susceptibility to osteoporosis in postmenopausal women. Gynecol Obstet Invest. 2003;55:82–87. doi: 10.1159/000070179. [DOI] [PubMed] [Google Scholar]
- 257.Braga V, Sangalli A, Malerba G, Mottes M, Mirandola S, Gatti D, Rossini M, Zamboni M, Adami S. Relationship among VDR (BsmI and FokI), COLIA1, and CTR polymorphisms with bone mass, bone turnover markers, and sex hormones in men. Calcif Tissue Int. 2002;70:457–462. doi: 10.1007/s00223-001-1088-9. [DOI] [PubMed] [Google Scholar]
- 258.Somner J, McLellan S, Cheung J, Mak YT, Frost ML, Knapp KM, Wierzbicki AS, Wheeler M, Fogelman I, Ralston SH, Hampson GN. Polymorphisms in the P450 c17 (17-hydroxylase/17,20-Lyase) and P450 c19 (aromatase) genes: Association with serum sex steroid concentrations and bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2004;89:344–351. doi: 10.1210/jc.2003-030164. [DOI] [PubMed] [Google Scholar]
- 259.Zarrabeitia MT, Hernandez JL, Valero C, Zarrabeitia AL, Garcia-Unzueta M, Amado JA, Gonzalez-Macias J, Riancho JA. A common polymorphism in the 5′-untranslated region of the aromatase gene influences bone mass and fracture risk. Eur J Endocrinol. 2004;150:699–704. doi: 10.1530/eje.0.1500699. [DOI] [PubMed] [Google Scholar]
- 260.Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A, Mavilia C, Del Monte F, Gonnelli S, Lucani B, Gennari C, Brandi ML. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: Effects on bone metabolism. J Clin Endocrinol Metab. 2004;89:2803–2810. doi: 10.1210/jc.2003-031342. [DOI] [PubMed] [Google Scholar]
- 261.Salmen T, Heikkinen AM, Mahonen A, Kroger H, Komulainen M, Pallonen H, Saarikoski S, Honkanen R, Maenpaa PH. Relation of aromatase gene polymorphism and hormone replacement therapy to serum estradiol levels, bone mineral density, and fracture risk in early postmenopausal women. Ann Med. 2003;35:282–288. doi: 10.1080/07853890310006370. [DOI] [PubMed] [Google Scholar]
- 262.Van Pottelbergh I, Goemaere S, Kaufman JM. Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. J Clin Endocrinol Metab. 2003;88:3075–3081. doi: 10.1210/jc.2002-021691. [DOI] [PubMed] [Google Scholar]
- 263.Lorentzon M, Swanson C, Erikson A, Ohlsson C. Aromatase CYP19 genetic polymorphism is associated with adult stature, androgen levels, and bone mineral density in 18–20-year-old Swedish males. J Bone Miner Res. 2004;19:S382. [Google Scholar]
- 264.Aloanzi ZH, Tuck SP, Varanasi SS, Raj N, Harrop JS, Summers GD, Cook DB, Francis RM, Datta HK. (TAAA)(n)-Alu element polymorphism in vitamin D binding protein gene and its association with bone density and osteoporosis in men. J Bone Miner Res. 2004;19:S249. [Google Scholar]
- 265.Ongphiphadhanakul B, Chanprasertyothin S, Payattikul P, Saetung S, Rajatanavin R. The implication of assessing a polymorphism in estrogen receptor alpha gene in the risk assessment of osteoporosis using a screening tool for osteoporosis in Asians. Osteoporos Int. 2003;14:863–867. doi: 10.1007/s00198-003-1464-6. [DOI] [PubMed] [Google Scholar]
- 266.Fountas L, Anapliotou M, Kominakis A, Sekeris CE, Kassi E, Moutsatsou P. Estrogen receptor alpha gene analysis in osteoporosis and familial osteoporosis. Osteoporos Int. 2004;15:948–956. doi: 10.1007/s00198-004-1654-x. [DOI] [PubMed] [Google Scholar]
- 267.Allcroft LC, Varanasi SS, Dimopoulos D, Francis RM, Datta HK. Mutational and polymorphic analysis of the estradiol receptor-alpha gene in men with symptomatic vertebral fractures. Calcif Tissue Int. 2002;71:400–405. doi: 10.1007/s00223-001-2040-8. [DOI] [PubMed] [Google Scholar]
- 268.Zmuda JM, Cauley JA, Lui L, Greene DR, Li J, Walker K, Stone KL, Ensrud KE, Hillier TA, Peltz G, Cummings SR. Estrogen receptor-a genotype and hip fracture risk in older women: Evidence for a strong interaction with body mass index. J Bone Miner Res. 2004;19:S387. [Google Scholar]
- 269.Suuriniemi M, Mahonen A, Kovanen V, Alen M, Lyytikainen A, Wang Q, Kroger H, Cheng S. Association between exercise and pubertal BMD is modulated by estrogen receptor alpha genotype. J Bone Miner Res. 2004;19:1758–1765. doi: 10.1359/JBMR.040918. [DOI] [PubMed] [Google Scholar]
- 270.Long JR, Zhang YY, Liu PY, Liu YJ, Shen H, Dvornyk V, Zhao LJ, Deng HW. Association of estrogen receptor alpha and vitamin D receptor gene polymorphisms with bone mineral density in Chinese males. Calcif Tissue Int. 2004;74:270–276. doi: 10.1007/s00223-003-0087-4. [DOI] [PubMed] [Google Scholar]
- 271.Sapir-Koren R, Livshits G, Kobyliansky E. Genetic effects of estrogen receptor alpha and collagen IA1 genes on the relationships of parathyroid hormone and 25 hydroxyvitamin D with bone mineral density in Caucasian women. Metabolism. 2003;52:1129–1135. doi: 10.1016/s0026-0495(03)00187-2. [DOI] [PubMed] [Google Scholar]
- 272.Zhang YY, Long JR, Liu PY, Liu YJ, Shen H, Zhao LJ, Deng HW. Estrogen receptor alpha and vitamin D receptor gene polymorphisms and bone mineral density: Association study of healthy pre- and postmenopausal Chinese women. Biochem Biophys Res Commun. 2003;308:777–783. doi: 10.1016/s0006-291x(03)01479-7. [DOI] [PubMed] [Google Scholar]
- 273.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, Mavilia C, Del Monte F, Melton LJ, III, Brandi ML. Relationship of estrogen receptor genotypes to bone mineral density and to rates of bone loss in men. J Clin Endocrinol Metab. 2004;89:1808–1816. doi: 10.1210/jc.2003-031448. [DOI] [PubMed] [Google Scholar]
- 274.Liu J, Zhu H, Zhu X, Dai M, Jiang L, Xu M, Chen J. Estrogen receptor gene polymorphisms and bone mineral density in Chinese postmenopausal women. Chin Med J (Engl) 2003;116:364–367. [PubMed] [Google Scholar]
- 275.Boot AM, van der Sluis IM, de Muinck Keizer-Schrama SM, van Meurs JB, Krenning EP, Pols HA, Uitterlinden AG. Estrogen receptor alpha gene molymorphisms and bone mineral density in healthy children and young adults. Calcif Tissue Int. 2004;74:495–500. doi: 10.1007/s00223-003-0168-4. [DOI] [PubMed] [Google Scholar]
- 276.Koh JM, Nam-Goong IS, Hong JS, Kim HK, Kim JS, Kim SY, Kim GS. Oestrogen receptor alpha genotype, and interactions between vitamin D receptor and transforming growth factor-beta1 genotypes are associated with quantitative calcaneal ultrasound in postmenopausal women. Clin Endocrinol (Oxf) 2004;60:232–240. doi: 10.1046/j.1365-2265.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- 277.Matsushita H, Kurabayashi T, Tomita M, Tanaka K. Effects of vitamin D and estrogen receptor gene polymorphisms on the changes in lumbar bone mineral density with multiple pregnancies in Japanese women. Hum Reprod. 2004;19:59–64. doi: 10.1093/humrep/deh009. [DOI] [PubMed] [Google Scholar]
- 278.Valimaki VV, Piippo K, Valimaki S, Loyttyniemi E, Kontula K, Valimaki MJ. Relation of the XbaI and PvuII polymorphisms of the estrogen receptor gene and the CAG repeat polymorphism of the androgen receptor gene to peak bone mass and bone turnover rate among young healthy men. J Bone Miner Res. 2004;19:S247. doi: 10.1007/s00198-005-1889-1. [DOI] [PubMed] [Google Scholar]
- 279.Masi L, Ottanelli S, Del Monte F, Carbonell S, Guazzini L, Fossi N, Gozzini A, Mavilla C, Falchetti A, Imbriaco R, Tanini A, Brandi ML. Aromatase and estrogen receptor alpha gene polymorhpisms: Response of the bone mineral density in postmenopausal women to HRT. J Bone Miner Res. 2004;19:S248–S249. [Google Scholar]
- 280.Zhang Z, Qin Y, Huang Q, He J, Li M, Zhou Q, Liu Y, Hu H. Association of estrogen receptor-alpha and vitamin D receptor genotypes with therapeutic response to calcium in postmenopausal Chinese women. J Bone Miner Res. 2004;19:S246. [PubMed] [Google Scholar]
- 281.Kung AWC, Lau HHL, Paterson AD, Cheung WMW, Luk KDK, Chan V. Test of linkage and/or association of genes and bone mineral density in Chinese. J Bone Miner Res. 2004;19:S243. [Google Scholar]
- 282.Scariano JK, Simplicio SG, Montoya GD, Garry PJ, Baumgartner RN. Estrogen receptor beta dinucleotide (CA) repeat polymorphism is significantly associated with bone mineral density in postmenopausal women. Calcif Tissue Int. 2004;74:501–508. doi: 10.1007/s00223-003-0170-x. [DOI] [PubMed] [Google Scholar]
- 283.Lau HH, Ho AY, Luk KD, Kung AW. Estrogen receptor beta gene polymorphisms are associated with higher bone mineral density in premenopausal, but not postmenopausal southern Chinese women. Bone. 2002;31:276–281. doi: 10.1016/s8756-3282(02)00827-x. [DOI] [PubMed] [Google Scholar]
- 284.Arko B, Prezelj J, Komel R, Kocijancic A, Marc J. No major effect of estrogen receptor beta gene RsaI polymorphism on bone mineral density and response to alendronate therapy in postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2002;81:147–152. doi: 10.1016/s0960-0760(02)00061-4. [DOI] [PubMed] [Google Scholar]
- 285.Liu X, Rosen C, Mora S, Dong D, Pitukcheewanont P, Gilsanz V. Low DXA and CT bone measures in young adults with a simple sequence repeat in IGF-I gene. J Bone Miner Res. 2004;19:S248. [Google Scholar]
- 286.Han KO, Choi JT, Moon IG, Jeong MS, Yim CH, Chung HY, Jang HC, Yoon HK, Han IK. Nonassociation of interleukin-1 receptor antagonist genotypes with bone mineral density, bone turnover status, and estrogen responsiveness in Korean postmenopausal women. Bone. 2002;31:612–615. doi: 10.1016/s8756-3282(02)00873-6. [DOI] [PubMed] [Google Scholar]
- 287.Nordstrom A, Gerdhem P, Brandstrom H, Stiger F, Lerner UH, Lorentzon M, Obrant K, Nordstrom P, Akesson K. Interleukin-6 promoter polymorphism is associated with bone quality assessed by calcaneus ultrasound and previous fractures in a cohort of 75-year-old women. Osteoporos Int. 2004;15:820–826. doi: 10.1007/s00198-004-1610-9. [DOI] [PubMed] [Google Scholar]
- 288.Dhamrait SS, James L, Brull DJ, Myerson S, Hawe E, Pennell DJ, World M, Humphries SE, Haddad F, Montgomery HE. Cortical bone resorption during exercise is interleukin-6 genotype-dependent. Eur J Appl Physiol. 2003;89:21–25. doi: 10.1007/s00421-002-0750-x. [DOI] [PubMed] [Google Scholar]
- 289.Chung HW, Seo JS, Hur SE, Kim HL, Kim JY, Jung JH, Kim LH, Park BL, Shin HD. Association of interleukin-6 promoter variant with bone mineral density in premenopausal women. J Hum Genet. 2003;48:243–248. doi: 10.1007/s10038-003-0020-8. [DOI] [PubMed] [Google Scholar]
- 290.Ferrari SL, Ahn-Luong L, Garnero P, Humphries SE, Greenspan SL. Two promoter polymorphisms regulating interleukin-6 gene expression are associated with circulating levels of C-reactive protein and markers of bone resorption in postmenopausal women. J Clin Endocrinol Metab. 2003;88:255–259. doi: 10.1210/jc.2002-020092. [DOI] [PubMed] [Google Scholar]
- 291.Ishida R, Emi M, Ezura Y, Iwasaki H, Yoshida H, Suzuki T, Hosoi T, Inoue S, Shiraki M, Ito H, Orimo H. Association of a haplotype (196Phe/532Ser) in the interleukin-1-receptor-associated kinase (IRAK1) gene with low radial bone mineral density in two independent populations. J Bone Miner Res. 2003;18:419–423. doi: 10.1359/jbmr.2003.18.3.419. [DOI] [PubMed] [Google Scholar]
- 292.Ogata N, Matsumura Y, Shiraki M, Kawano K, Koshizuka Y, Hosoi T, Nakamura K, Kuro O, Kawaguchi H. Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone. 2002;31:37–42. doi: 10.1016/s8756-3282(02)00786-x. [DOI] [PubMed] [Google Scholar]
- 293.Koay MA, Woon PY, Zhang Y, Miles LJ, Duncan EL, Ralston SH, Compston JE, Cooper C, Keen R, Langdahl BL, MacLelland A, O’Riordan J, Pols HA, Reid DM, Uitterlinden AG, Wass JA, Brown MA. Influence of LRP5 polymorphisms on normal variation in BMD. J Bone Miner Res. 2004;19:1619–1627. doi: 10.1359/JBMR.040704. [DOI] [PubMed] [Google Scholar]
- 294.Golbahar J, Hamidi A, Aminzadeh MA, Omrani GR. Association of plasma folate, plasma total homocysteine, but not methylenetetrahydrofolate reductase C667T polymorphism, with bone mineral density in postmenopausal Iranian women: A cross-sectional study. Bone. 2004;35:760–765. doi: 10.1016/j.bone.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 295.Villadsen MM, Bunger MH, Carstens M, Stenkjaer L, Langdahl BL. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism is associated with osteoporotic vertebral fractures, but is a weak predictor of BMD. Osteoporos Int. 2004;16:411–416. doi: 10.1007/s00198-004-1704-4. [DOI] [PubMed] [Google Scholar]
- 296.Jorgensen HL, Madsen JS, Madsen B, Saleh MM, Abrahamsen B, Fenger M, Lauritzen JB. Association of a common allelic polymorphism (C677T) in the methylene tetrahydrofolate reductase gene with a reduced risk of osteoporotic fractures. A case control study in Danish postmenopausal women. Calcif Tissue Int. 2002;71:386–392. doi: 10.1007/s00223-001-2126-3. [DOI] [PubMed] [Google Scholar]
- 297.Merlotti D, Gennari L, Martini G, Calabr A, De Paola V, Dal Canto N, Salvadori S, Valenti R, Franci B, Campagna S, Lucani B, Nuti R. OPG polymorphism, serum RANKI, serum OPG and bone metabolism in men. J Bone Miner Res. 2004;19:S438. [Google Scholar]
- 298.Brandstrom H, Gerdhem P, Stiger F, Obrant KJ, Melhus H, Ljunggren O, Kindmark A, Akesson K. Single nucleotide polymorphisms in the human gene for osteoprotegerin are not related to bone mineral density or fracture in elderly women. Calcif Tissue Int. 2004;74:18–24. doi: 10.1007/s00223-002-2136-9. [DOI] [PubMed] [Google Scholar]
- 299.Ohmori H, Makita Y, Funamizu M, Hirooka K, Hosoi T, Orimo H, Suzuki T, Ikari K, Nakajima T, Inoue I, Hata A. Linkage and association analyses of the osteoprotegerin gene locus with human osteoporosis. J Hum Genet. 2002;47:400–406. doi: 10.1007/s100380200058. [DOI] [PubMed] [Google Scholar]
- 300.Katsumata K, Nishizawa K, Unno A, Fujita Y, Tokita A. Association of gene polymorphisms and bone density in Japanese girls. J Bone Miner Metab. 2002;20:164–169. doi: 10.1007/s007740200023. [DOI] [PubMed] [Google Scholar]
- 301.Scillitani A, Jang C, Wong B, Rubin L, Hendy G, Cole D. A functional (AAAG)n polymorphism in the PTHR1 promoter is associated with height but not with BMD in a large cohort of young Caucasian women. J Bone Miner Res. 2004;19:S248. doi: 10.1007/s00439-006-0155-8. [DOI] [PubMed] [Google Scholar]
- 302.Ezura Y, Kajita M, Ishida R, Yoshida S, Yoshida H, Suzuki T, Hosoi T, Inoue S, Shiraki M, Orimo H, Emi M. Association of multiple nucleotide variations in the pituitary glutaminyl cyclase gene (QPCT) with low radial BMD in adult women. J Bone Miner Res. 2004;19:1296–1301. doi: 10.1359/JBMR.040324. [DOI] [PubMed] [Google Scholar]
- 303.Lau HH, Ho AY, Luk KD, Kung AW. Transforming growth factor-beta1 gene polymorphisms and bone turnover, bone mineral density and fracture risk in southern Chinese women. Calcif Tissue Int. 2004;74:516–521. doi: 10.1007/s00223-004-0163-4. [DOI] [PubMed] [Google Scholar]
- 304.Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF. Polymorphisms in the transforming growth factor beta 1 gene and osteoporosis. Bone. 2003;32:297–310. doi: 10.1016/s8756-3282(02)00971-7. [DOI] [PubMed] [Google Scholar]
- 305.Tasker PN, Albagha OM, Masson CB, Reid DM, Ralston SH. Association between TNFRSF1B polymorphisms and bone mineral density, bone loss and fracture. Osteoporos Int. 2004;15:903–908. doi: 10.1007/s00198-004-1617-2. [DOI] [PubMed] [Google Scholar]
- 306.Furuta I, Kobayashi N, Fujino T, Kobamatsu Y, Shirogane T, Yaegashi M, Sakuragi N, Cho K, Yamada H, Okuyama K, Minakami H. Bone mineral density of the lumbar spine is associated with TNF gene polymorphisms in early postmenopausal Japanese women. Calcif Tissue Int. 2004;74:509–515. doi: 10.1007/s00223-003-0105-6. [DOI] [PubMed] [Google Scholar]
- 307.Wennberg P, Nordstrom P, Lorentzon R, Lerner UH, Lorentzon M. TNF-alpha gene polymorphism and plasma TNF-alpha levels are related to lumbar spine bone area in healthy female Caucasian adolescents. Eur J Endocrinol. 2002;146:629–634. doi: 10.1530/eje.0.1460629. [DOI] [PubMed] [Google Scholar]
- 308.Ota N, Nakajima T, Ezura Y, Iwasaki H, Suzuki T, Hosoi T, Orimo H, Inoue S, Ito H, Emi M. Association of a single nucleotide variant in the human tumour necrosis factor alpha promoter region with decreased bone mineral density. Ann Hum Biol. 2002;29:550–558. doi: 10.1080/03014460210135730. [DOI] [PubMed] [Google Scholar]
- 309.Qin YJ, Zhang ZL, Huang QR, He JM, Hu YQ, Zhao Q, Lu JH, Li M, Liu YJ. Association of vitamin D receptor and estrogen receptor-alpha gene polymorphism with peak bone mass and bone size in Chinese women. Acta Pharmacol Sin. 2004;25:462–468. [PubMed] [Google Scholar]
- 310.Huang QR, Zhang ZL, Qin YJ, He JW, Lu JH, Zhou Q, Hu YQ, Li M, Liu YJ. Association of Apa I polymorphism of vitamin D receptor gene with bone mass in men. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:254–257. [PubMed] [Google Scholar]
- 311.Zajickova K, Zofkova I, Bahbouh R, Krepelova A. Vitamin D receptor gene polymorphisms, bone mineral density and bone turnover: FokI genotype is related to postmenopausal bone mass. Physiol Res. 2002;51:501–509. [PubMed] [Google Scholar]
- 312.Kim JG, Kwon JH, Kim SH, Choi YM, Moon SY, Lee JY. Association between vitamin D receptor gene haplotypes and bone mass in postmenopausal Korean women. Am J Obstet Gynecol. 2003;189:1234–1240. doi: 10.1067/s0002-9378(03)00650-1. [DOI] [PubMed] [Google Scholar]
- 313.Taguchi A, Whit SC, Kobayashi J, Survice SK, Nakamoto T, Ohtsuka M, Sanada M, Tsuda M, Ohama K, Suei Y, Tanimoto K. Relationship between vitamin D receptor gene polymorphism and trabecular bone pattern of the mandible in Japanese postmenopausal wome. J Bone Miner Res. 2004;19:S245. [Google Scholar]
- 314.Rogers A, Clowes JA, Gossiel F, Peel NFA, Eastell R. The effect of BsmI and FokI genotypes on BMD and bone turnover response to raloxifene therapy. J Bone Miner Res. 2004;19:S131. [Google Scholar]
- 315.Rapuri PB, Gallagher JC, Knezetic JA, Kinyamu HK, Ryschon KL. Association between Vitamin D receptor polymorphisms and the rate of bone loss in elderly women-importance of adjusting for dietary and lifestyle factors. J Steroid Biochem Mol Biol. 2004;89–90:503–506. doi: 10.1016/j.jsbmb.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 316.Obermayer-Pietsch BM, Lange U, Tauber G, Fruhauf G, Fahrleitner A, Dobnig H, Hermann J, Aglas F, Teichmann J, Neeck G, Leb G. Vitamin D receptor initiation codon polymorphism, bone density and inflammatory activity of patients with ankylosing spondylitis. Osteoporos Int. 2003;14:995–1000. doi: 10.1007/s00198-003-1501-5. [DOI] [PubMed] [Google Scholar]
- 317.Palomba S, Numis FG, Mossetti G, Rendina D, Vuotto P, Russo T, Zullo F, Nappi C, Nunziata V. Effectiveness of alendronate treatment in postmenopausal women with osteoporosis: Relationship with BsmI vitamin D receptor genotypes. Clin Endocrinol (Oxf) 2003;58:365–371. doi: 10.1046/j.1365-2265.2003.01724.x. [DOI] [PubMed] [Google Scholar]
- 318.Palomba S, Numis FG, Mossetti G, Rendina D, Vuotto P, Russo T, Zullo F, Nappi C, Nunziata V. Raloxifene administration in postmenopausal women with osteoporosis: Effect of different BsmI vitamin D receptor genotypes. Hum Reprod. 2003;18:192–198. doi: 10.1093/humrep/deg031. [DOI] [PubMed] [Google Scholar]
- 319.Pinter B, Kocijancic A, Marc J, Andolsek-Jeras L, Prezelj J. Vitamin D receptor gene polymorphism and bone metabolism during low-dose oral contraceptive use in young women. Contraception. 2003;67:33–37. doi: 10.1016/s0010-7824(02)00432-8. [DOI] [PubMed] [Google Scholar]
- 320.Laaksonen M, Karkkainen M, Outila T, Vanninen T, Ray C, Lamberg-Allardt C. Vitamin D receptor gene BsmI-polymorphism in Finnish premenopausal and postmenopausal women: Its association with bone mineral density, markers of bone turnover, and intestinal calcium absorption, with adjustment for lifestyle factors. J Bone Miner Metab. 2002;20:383–390. doi: 10.1007/s007740200055. [DOI] [PubMed] [Google Scholar]
- 321.Blanchet C, Giguere Y, Prud’homme D, Dumont M, Rousseau F, Dodin S. Association of physical activity and bone: Influence of vitamin D receptor genotype. Med Sci Sports Exerc. 2002;34:24–31. doi: 10.1097/00005768-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 322.Quesada JM, Casado A, Diaz C, Barrios L, Cuenca-Acevedo R, Dorado G. Allele-frequency determination of BsmI and FokI polymorphisms of the VDR gene by quantitative real-time PCR (QRT-PCR) in pooled genomic DNA samples. J Steroid Biochem Mol Biol. 2004;89–90:209–214. doi: 10.1016/j.jsbmb.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 323.Douroudis K, Tarassi K, Ioannidis G, Giannakopoulos F, Moutsatsou P, Thalassinos N, Papasteriades C. Association of vitamin D receptor gene polymorphisms with bone mineral density in postmenopausal women of Hellenic origin. Maturitas. 2003;45:191–197. doi: 10.1016/s0378-5122(03)00148-8. [DOI] [PubMed] [Google Scholar]
- 324.Quesada-Gomez JM, Casado A, Cuenca-Acevedo R, Barrios L, Diaz-Molina C, Dorado G. The polymorphisms of the vdr cdx-2 influence bone mineral density (BMD) in postmenopausal women. J Bone Miner Res. 2004;19:S250. [Google Scholar]
- 325.Nakamura O, Ishii T, Ando Y, Amagai H, Oto M, Imafuji T, Tokuyama K. Potential role of vitamin D receptor gene polymorphism in determining bone phenotype in young male athletes. J Appl Physiol. 2002;93:1973–1979. doi: 10.1152/japplphysiol.00663.2002. [DOI] [PubMed] [Google Scholar]
- 326.Zhang ZL, Zhao JX, Meng XW, Zhou XY, Xing XP, Xia WB. Association of polymorphisms of vitamin D receptor gene start codon and 3′-end region with bone mineral density in postmenopausal women. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2003;20:5–8. [PubMed] [Google Scholar]
- 327.Laaksonen MM, Karkkainen MU, Outila TA, Rita HJ, Lamberg-Allardt CJ. Vitamin D receptor gene start codon polymorphism (FokI) is associated with forearm bone mineral density and calcaneal ultrasound in Finnish adolescent boys but not in girls. J Bone Miner Metab. 2004;22:479–485. doi: 10.1007/s00774-004-0510-6. [DOI] [PubMed] [Google Scholar]
- 328.Strandberg S, Nordstrom P, Lorentzon R, Lorentzon M. Vitamin D receptor start codon polymorphism (FokI) is related to bone mineral density in healthy adolescent boys. J Bone Miner Metab. 2003;21:109–113. doi: 10.1007/s007740300018. [DOI] [PubMed] [Google Scholar]
- 329.Lau EM, Lam V, Li M, Ho K, Woo J. Vitamin D receptor start codon polymorphism (Fok I) and bone mineral density in Chinese men and women. Osteoporos Int. 2002;13:218–221. doi: 10.1007/s001980200017. [DOI] [PubMed] [Google Scholar]
- 330.Vidal C, Grima C, Brincat M, Megally N, Xuereb-Anastasi A. Associations of polymorphisms in the vitamin D receptor gene (BsmI and FokI) with bone mineral density in postmenopausal women in Malta. Osteoporos Int. 2003;14:923–928. doi: 10.1007/s00198-003-1457-5. [DOI] [PubMed] [Google Scholar]
- 331.Grundberg E, Brandstrom H, Ribom EL, Ljunggren O, Kindmark A, Mallmin H. A poly adenosine repeat in the human vitamin D receptor gene is associated with bone mineral density in young Swedish women. Calcif Tissue Int. 2003;73:455–462. doi: 10.1007/s00223-002-0032-y. [DOI] [PubMed] [Google Scholar]
- 332.Rapuri PB, Gallagher JC, Haynatzka V. Poly andenosine repeat in the human vitamin D receptor gene is associated with increased bone loss in elderly women. J Bone Miner Res. 2004;19:S246. [Google Scholar]
- 333.Kurabayashi T, Matsushita H, Tomita M, Kato N, Kikuchi M, Nagata H, Honda A, Yahata T, Tanaka K. Association of vitamin D and estrogen receptor gene polymorphism with the effects of longterm hormone replacement therapy on bone mineral density. J Bone Miner Metab. 2004;22:241–247. doi: 10.1007/s00774-003-0474-y. [DOI] [PubMed] [Google Scholar]
- 334.Duman BS, Tanakol R, Erensoy N, Ozturk M, Yilmazer S. Vitamin D receptor alleles, bone mineral density and turnover in postmenopausal osteoporotic and healthy women. Med Princ Pract. 2004;13:260–266. doi: 10.1159/000079524. [DOI] [PubMed] [Google Scholar]
- 335.McClean E, Archbold GP, Taggart HM. Do the COL1A1 and Taq 1 vitamin D receptor polymorphisms have a role in identifying individuals at risk of developing osteoporosis? Ulster Med J. 2003;72:26–33. [PMC free article] [PubMed] [Google Scholar]
- 336.Van Pottelbergh I, Goemaere S, De Bacquer D, De Paepe A, Kaufman M. Vitamin D receptor gene allelic variants, bone density, and bone turnover in community-dwelling men. Bone. 2002;31:631–637. doi: 10.1016/s8756-3282(02)00867-0. [DOI] [PubMed] [Google Scholar]
- 337.Krall EA, Fleet JC, Vokonas PS, Miller DR, Rich SE. Interactions of calcium intake with FokI and androgen receptor genotypes in men. J Bone Miner Res. 2004;19:S245. [Google Scholar]
- 338.Zmuda JM, Cauley JA, Danielson ME, Ferrell RE. Vitamin D receptor and aromatase gene interaction and bone mass in older African-American women. Metabolism. 2003;52:521–523. doi: 10.1053/meta.2003.50089. [DOI] [PubMed] [Google Scholar]
- 339.Qin Y, Zhang Z, Huang Q, He J, Hu Y, Zhou Q, Li M, Liu Y. Association of peak bone mineral density and related factors with osteoporosis in Chinese women: A case control study for daughter to mother. J Bone Miner Res. 2004;19:S242. [Google Scholar]
- 340.Dacquin R, Davey RA, Laplace C, Levasseur R, Morris HA, Goldring SR, Gebre-Medhin S, Galson DL, Zajac JD, Karsenty G. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol. 2004;164:509–514. doi: 10.1083/jcb.200312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci USA. 2003;100:9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nielsen KL, Allen MR, Bloomfield SA, Andersen TL, Chen XD, Poulsen HS, Young MF, Heegaard AM. Biglycan deficiency interferes with ovariectomy-induced bone loss. J Bone Miner Res. 2003;18:2152–2158. doi: 10.1359/jbmr.2003.18.12.2152. [DOI] [PubMed] [Google Scholar]
- 343.Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, Hanks MC, Amling M, Pinero GJ, Harada S, Behringer RR. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J Biol Chem. 2004;279:27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- 344.Peng Z, Li X, Makela S, Vaananen HK, Poutanen M. Skeletal changes in transgenic male mice expressing human cytochrome p450 aromatase. J Bone Miner Res. 2004;19:1320–1328. doi: 10.1359/JBMR.040510. [DOI] [PubMed] [Google Scholar]
- 345.Lee KC, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and -beta. J Endocrinol. 2004;182:193–201. doi: 10.1677/joe.0.1820193. [DOI] [PubMed] [Google Scholar]
- 346.Roschger P, Matsuo K, Misof BM, Tesch W, Jochum W, Wagner EF, Fratzl P, Klaushofer K. Normal mineralization and nanostructure of sclerotic bone in mice overexpressing Fra-1. Bone. 2004;34:776–782. doi: 10.1016/j.bone.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 347.Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC. Megakaryocyteosteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res. 2004;19:652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- 348.Hamrick MW, Pennington C, Byron CD. Bone architecture and disc degeneration in the lumbar spine of mice lacking GDF-8 (myostatin) J Orthop Res. 2003;21:1025–1032. doi: 10.1016/S0736-0266(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 349.Levasseur R, Barrios R, Elefteriou F, Glass DA, Lieberman MW, Karsenty G. Reversible skeletal abnormalities in gamma-glutamyl transpeptidase-deficient mice. Endocrinology. 2003;144:2761–2764. doi: 10.1210/en.2002-0071. [DOI] [PubMed] [Google Scholar]
- 350.Wang L, Liu S, Quarles LD, Spurney RF. Targeted overexpression of G protein-coupled receptor kinase 2 (GRK2) in osteoblasts promotes bone loss. Am J Physiol Endocrinol Metab. 2004;288:E826–E834. doi: 10.1152/ajpendo.00422.2004. [DOI] [PubMed] [Google Scholar]
- 351.Justesen J, Mosekilde L, Holmes M, Stenderup K, Gasser J, Mullins JJ, Seckl JR, Kassem M. Mice deficient in 11beta-hydroxysteroid dehydrogenase type 1 lack bone marrow adipocytes, but maintain normal bone formation. Endocrinology. 2004;145:1916–1925. doi: 10.1210/en.2003-1427. [DOI] [PubMed] [Google Scholar]
- 352.Kenner L, Hoebertz A, Beil T, Keon N, Karreth F, Eferl R, Scheuch H, Szremska A, Amling M, Schorpp-Kistner M, Angel P, Wagner EF. Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J Cell Biol. 2004;164:613–623. doi: 10.1083/jcb.200308155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Bohlooly Y, Mahlapuu M, Andersen H, Astrand A, Hjorth S, Svensson L, Tornell J, Snaith MR, Morgan DG, Ohlsson C. Osteoporosis in MCHR1-deficient mice. Biochem Biophys Res Commun. 2004;318:964–969. doi: 10.1016/j.bbrc.2004.04.122. [DOI] [PubMed] [Google Scholar]
- 354.van’t Hof RJ, Macphee J, Libouban H, Helfrich MH, Ralston SH. Regulation of bone mass and bone turnover by neuronal nitric oxide synthase. Endocrinology. 2004;145:5068–5074. doi: 10.1210/en.2004-0205. [DOI] [PubMed] [Google Scholar]
- 355.Sainsbury A, Baldock PA, Schwarzer C, Ueno N, Enriquez RF, Couzens M, Inui A, Herzog H, Gardiner EM. Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Mol Cell Biol. 2003;23:5225–5233. doi: 10.1128/MCB.23.15.5225-5233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 357.Kitahara K, Ishijima M, Rittling SR, Tsuji K, Kurosawa H, Nifuji A, Denhardt DT, Noda M. Osteopontin deficiency induces parathyroid hormone enhancement of cortical bone formation. Endocrinology. 2003;144:2132–2140. doi: 10.1210/en.2002-220996. [DOI] [PubMed] [Google Scholar]
- 358.Hashimoto-Gotoh T, Ohnishi H, Tsujimura A, Tsunezuka H, Imai K, Masuda H, Nakamura T. Bone mass increase specific to the female in a line of transgenic mice overexpressing human osteoblast stimulating factor-1. J Bone Miner Metab. 2004;22:278–282. doi: 10.1007/s00774-003-0485-8. [DOI] [PubMed] [Google Scholar]
- 359.Daci E, Everts V, Torrekens S, Van HE, Tigchelaar-Gutterr W, Bouillon R, Carmeliet G. Increased bone formation in mice lacking plasminogen activators. J Bone Miner Res. 2003;18:1167–1176. doi: 10.1359/jbmr.2003.18.7.1167. [DOI] [PubMed] [Google Scholar]
- 360.VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest. 2003;112:1429–1436. doi: 10.1172/JCI19504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Chiusaroli R, Knobler H, Luxenburg C, Sanjay A, Granot-Attas S, Tiran Z, Miyazaki T, Harmelin A, Baron R, Elson A. Tyrosine phosphatase epsilon is a positive regulator of osteoclast function in vitro and in vivo. Mol Biol Cell. 2004;15:234–244. doi: 10.1091/mbc.E03-04-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Tsuchiya H, Kitoh H, Sugiura F, Ishiguro N. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2003;301:338–343. doi: 10.1016/s0006-291x(02)03026-7. [DOI] [PubMed] [Google Scholar]
- 363.Kim S, Koga T, Isobe M, Kern BE, Yokochi T, Chin YE, Karsenty G, Taniguchi T, Takayanagi H. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 2003;17:1979–1991. doi: 10.1101/gad.1119303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Geoffroy V, Marty-Morieux C, Le GN, Clement-Lacroix P, Terraz C, Frain M, Roux S, Rossert J, de Vernejoul MC. In vivo inhibition of osteoblastic metalloproteinases leads to increased trabecular bone mass. J Bone Miner Res. 2004;19:811–822. doi: 10.1359/JBMR.040119. [DOI] [PubMed] [Google Scholar]
- 365.Devoto M, Specchia C, Li HH, Caminis J, Tenenhouse A, Rodriguez H, Spotila LD. Variance component linkage analysis indicates a QTL for femoral neck bone mineral density on chromosome 1p36. Hum Mol Genet. 2001;10:2447–2452. doi: 10.1093/hmg/10.21.2447. [DOI] [PubMed] [Google Scholar]
- 366.Karasik D, Myers RH, Hannan MT, Gagnon D, McLean RR, Cupples LA, Kiel DP. Mapping of quantitative ultrasound of the calcaneus bone to chromosome 1 by genome-wide linkage analysis. Osteoporos Int. 2002;13:796–802. doi: 10.1007/s001980200110. [DOI] [PubMed] [Google Scholar]
- 367.Niu T, Chen C, Cordell H, Yang J, Wang B, Wang Z, Fang Z, Schork NJ, Rosen CJ, Xu X. A genome-wide scan for loci linked to forearm bone mineral density. Hum Genet. 1999;104:226–233. doi: 10.1007/s004390050940. [DOI] [PubMed] [Google Scholar]
- 368.Duncan EL, Brown MA, Sinsheimer J, Bell J, Carr AJ, Wordsworth BP, Wass JA. Suggestive linkage of the parathyroid receptor type 1 to osteoporosis. J Bone Miner Res. 1999;14:1993–1999. doi: 10.1359/jbmr.1999.14.12.1993. [DOI] [PubMed] [Google Scholar]
- 369.Ota N, Hunt SC, Nakajima T, Suzuki T, Hosoi T, Orimo H, Shirai Y, Emi M. Linkage of human tumor necrosis factor-alpha to human osteoporosis by sib pair analysis. Genes Immun. 2000;1:260–264. doi: 10.1038/sj.gene.6363668. [DOI] [PubMed] [Google Scholar]
- 370.Mitchell BD, Cole SA, Bauer RL, Iturria SJ, Rodriguez EA, Blangero J, MacCluer JW, Hixson JE. Genes influencing variation in serum osteocalcin concentrations are linked to markers on chromosomes 16q and 20q. J Clin Endocrinol Metab. 2000;85:1362–1366. doi: 10.1210/jcem.85.4.6571. [DOI] [PubMed] [Google Scholar]
- 371.Harris SE, Guo D, Harris MA, Krishnaswamy A, Lichtler A. Transcriptional regulation of BMP-2 activated genes in osteoblasts using gene expression microarray analysis: Role of Dlx2 and Dlx5 transcription factors. Front Biosci. 2003;8:s1249–s1265. doi: 10.2741/1170. [DOI] [PubMed] [Google Scholar]
- 372.Guillemot L, Hammar E, Kaister C, Ritz J, Caille D, Jond L, Bauer C, Meda P, Citi S. Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression. J Cell Sci. 2004;117:5245–5256. doi: 10.1242/jcs.01399. [DOI] [PubMed] [Google Scholar]
- 373.von Schroeder HP, Veillette CJ, Payandeh J, Qureshi A, Heersche JN. Endothelin-1 promotes osteoprogenitor proliferation and differentiation in fetal rat calvarial cell cultures. Bone. 2003;33:673–684. doi: 10.1016/s8756-3282(03)00215-1. [DOI] [PubMed] [Google Scholar]
- 374.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 375.Kevorkian L, Young DA, Darrah C, Donell ST, Shepstone L, Porter S, Brockbank SM, Edwards DR, Parker AE, Clark IM. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- 376.Tardif G, Hum D, Pelletier JP, Boileau C, Ranger P, Martel-Pelletier J. Differential gene expression and regulation of the bone morphogenetic protein antagonists follistatin and gremlin in normal and osteoarthritic human chondrocytes and synovial fibroblasts. Arthritis Rheum. 2004;50:2521–2530. doi: 10.1002/art.20441. [DOI] [PubMed] [Google Scholar]
- 377.Shi J, Schmitt-Talbot E, DiMattia DA, Dullea RG. The differential effects of IL-1 and TNF-alpha on proinflammatory cytokine and matrix metalloproteinase expression in human chondrosarcoma cells. Inflamm Res. 2004;53:377–389. doi: 10.1007/s00011-004-1271-3. [DOI] [PubMed] [Google Scholar]
- 378.Elliott SF, Coon CI, Hays E, Stadheim TA, Vincenti MP. Bcl-3 is an interleukin-1-responsive gene in chondrocytes and synovial fibroblasts that activates transcription of the matrix metalloproteinase 1 gene. Arthritis Rheum. 2002;46:3230–3239. doi: 10.1002/art.10675. [DOI] [PubMed] [Google Scholar]
- 379.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and BMP signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 380.van der Pouw Kraan TC, van Gaalen FA, Kasperkovitz PV, Verbeet NL, Smeets TJ, Kraan MC, Fero M, Tak PP, Huizinga TW, Pieterman E, Breedveld FC, Alizadeh AA, Verweij CL. Rheumatoid arthritis is a heterogeneous disease: Evidence for differences in the activation of the STAT-1 pathway between rheumatoid tissues. Arthritis Rheum. 2003;48:2132–2145. doi: 10.1002/art.11096. [DOI] [PubMed] [Google Scholar]
- 381.Ohara N, Hayashi Y, Yamada S, Kim SK, Matsunaga T, Yanagiguchi K, Ikeda T. Early gene expression analyzed by cDNA microarray and RT-PCR in osteoblasts cultured with water-soluble and low molecular chitooligosaccharide. Biomaterials. 2004;25:1749–1754. doi: 10.1016/j.biomaterials.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 382.Zhang H, Liew CC, Marshall KW. Microarray analysis reveals the involvement of beta-2 microglobulin (B2M) in human osteoarthritis. Osteoarthritis Cartilage. 2002;10:950–960. doi: 10.1053/joca.2002.0850. [DOI] [PubMed] [Google Scholar]
- 383.Islam S, Kermode T, Sultana D, Moskowitz RW, Mukhtar H, Malemud CJ, Goldberg VM, Haqqi TM. Expression profile of protein tyrosine kinase genes in human osteoarthritis chondrocytes. Osteoarthritis Cartilage. 2001;9:684–693. doi: 10.1053/joca.2001.0465. [DOI] [PubMed] [Google Scholar]
- 384.Huh SJ, Paik DJ, Chung HS, Youn J. Regulation of GRB2 and FLICE2 expression by TNF-alpha in rheumatoid synovium. Immunol Lett. 2003;90:93–96. doi: 10.1016/j.imlet.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 385.Gallagher J, Howlin J, McCarthy C, Murphy EP, Bresnihan B, FitzGerald O, Godson C, Brady HR, Martin F. Identification of Naf1/ABIN-1 among TNF-alpha-induced expressed genes in human synoviocytes using oligonucleotide microarrays. FEBS Lett. 2003;551:8–12. doi: 10.1016/s0014-5793(03)00823-8. [DOI] [PubMed] [Google Scholar]
- 386.Takeoka Y, Kenny TP, Yago H, Naiki M, Gershwin ME, Robbins DL. Developmental considerations of sperm protein 17 gene expression in rheumatoid arthritis synoviocytes. Dev Immunol. 2002;9:97–102. doi: 10.1080/1044667021000095186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Sakurai D, Yamaguchi A, Tsuchiya N, Yamamoto K, Tokunaga K. Expression of ID family genes in the synovia from patients with rheumatoid arthritis. Biochem Biophys Res Commun. 2001;284:436–442. doi: 10.1006/bbrc.2001.4974. [DOI] [PubMed] [Google Scholar]
- 388.de Jong DS, Vaes BL, Dechering KJ, Feijen A, Hendriks JM, Wehrens R, Mummery CL, van Zoelen EJ, Olijve W, Steegenga WT. Identification of novel regulators associated with early-phase osteoblast differentiation. J Bone Miner Res. 2004;19:947–958. doi: 10.1359/JBMR.040216. [DOI] [PubMed] [Google Scholar]
- 389.de Jong DS, Steegenga WT, Hendriks JM, van Zoelen EJ, Olijve W, Dechering KJ. Regulation of Notch signaling genes during BMP2-induced differentiation of osteoblast precursor cells. Biochem Biophys Res Commun. 2004;320:100–107. doi: 10.1016/j.bbrc.2004.05.150. [DOI] [PubMed] [Google Scholar]
- 390.Gu K, Zhang L, Jin T, Rutherford RB. Identification of potential modifiers of Runx2/Cbfa1 activity in C2C12 cells in response to bone morphogenetic protein-7. Cells Tissues Organs. 2004;176:28–40. doi: 10.1159/000075025. [DOI] [PubMed] [Google Scholar]
- 391.Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W, Luu HH, Park JY, Haydon RC, He TC. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90:1149–1165. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- 392.Korchynskyi O, Dechering KJ, Sijbers AM, Olijve W, ten Dijke P. Gene array analysis of bone morphogenetic protein type I receptor-induced osteoblast differentiation. J Bone Miner Res. 2003;18:1177–1185. doi: 10.1359/jbmr.2003.18.7.1177. [DOI] [PubMed] [Google Scholar]
- 393.Balint E, Lapointe D, Drissi H, van der MC, Young DW, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Phenotype discovery by gene expression profiling: Mapping of biological processes linked to BMP-2-mediated osteoblast differentiation. J Cell Biochem. 2003;89:401–426. doi: 10.1002/jcb.10515. [DOI] [PubMed] [Google Scholar]
- 394.de Jong DS, van Zoelen EJ, Bauerschmidt S, Olijve W, Steegenga WT. Microarray analysis of bone morphogenetic protein, transforming growth factor beta, and activin early response genes during osteoblastic cell differentiation. J Bone Miner Res. 2002;17:2119–2129. doi: 10.1359/jbmr.2002.17.12.2119. [DOI] [PubMed] [Google Scholar]
- 395.Vaes BL, Dechering KJ, Feijen A, Hendriks JM, Lefevre C, Mummery CL, Olijve W, van Zoelen EJ, Steegenga WT. Comprehensive microarray analysis of bone morphogenetic protein 2-induced osteoblast differentiation resulting in the identification of novel markers for bone development. J Bone Miner Res. 2002;17:2106–2118. doi: 10.1359/jbmr.2002.17.12.2106. [DOI] [PubMed] [Google Scholar]
- 396.von Stechow D, Zurakowski D, Pettit AR, Muller R, Gronowicz G, Chorev M, Otu H, Libermann T, Alexander JM. Differential transcriptional effects of PTH and estrogen during anabolic bone formation. J Cell Biochem. 2004;93:476–490. doi: 10.1002/jcb.20174. [DOI] [PubMed] [Google Scholar]
- 397.Bourne S, Polak JM, Hughes SP, Buttery LD. Osteogenic differentiation of mouse embryonic stem cells: Differential gene expression analysis by cDNA microarray and purification of osteoblasts by cadherin-11 magnetically activated cell sorting. Tissue Eng. 2004;10:796–806. doi: 10.1089/1076327041348293. [DOI] [PubMed] [Google Scholar]
- 398.Lindberg MK, Moverare S, Eriksson AL, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Identification of estrogen-regulated genes of potential importance for the regulation of trabecular bone mineral density. J Bone Miner Res. 2002;17:2183–2195. doi: 10.1359/jbmr.2002.17.12.2183. [DOI] [PubMed] [Google Scholar]
- 399.Leclerc N, Luppen CA, Ho VV, Nagpal S, Hacia JG, Smith E, Frenkel B. Gene expression profiling of glucocorticoid-inhibited osteoblasts. J Mol Endocrinol. 2004;33:175–193. doi: 10.1677/jme.0.0330175. [DOI] [PubMed] [Google Scholar]
- 400.Eelen G, Verlinden L, van Camp M, van Hummelen P, Marchal K, de Moor B, Mathieu C, Carmeliet G, Bouillon R, Verstuyf A. The effects of 1alpha,25-dihydroxyvitamin D3 on the expression of DNA replication genes. J Bone Miner Res. 2004;19:133–146. doi: 10.1359/JBMR.0301204. [DOI] [PubMed] [Google Scholar]
- 401.Beck GR, Jr, Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res. 2003;288:288–300. doi: 10.1016/s0014-4827(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 402.Raouf A, Ganss B, McMahon C, Vary C, Roughley PJ, Seth A. Lumican is a major proteoglycan component of the bone matrix. Matrix Biol. 2002;21:361–367. doi: 10.1016/s0945-053x(02)00027-6. [DOI] [PubMed] [Google Scholar]
- 403.Huang W, Carlsen B, Rudkin GH, Shah N, Chung C, Ishida K, Yamaguchi DT, Miller TA. Effect of serial passage on gene expression in MC3T3-E1 preosteoblastic cells: A microarray study. Biochem Biophys Res Commun. 2001;281:1120–1126. doi: 10.1006/bbrc.2001.4458. [DOI] [PubMed] [Google Scholar]
- 404.Carinci F, Piattelli A, Stabellini G, Palmieri A, Scapoli L, Laino G, Caputi S, Pezzetti F. Calcium sulfate: Analysis of MG63 osteoblast-like cell response by means of a microarray technology. J Biomed Mater Res. 2004;71B:260–267. doi: 10.1002/jbm.b.30133. [DOI] [PubMed] [Google Scholar]
- 405.Carinci F, Pezzetti F, Volinia S, Laino G, Arcelli D, Caramelli E, Degidi M, Piattelli A. P-15 cell-binding domain derived from collagen: Analysis of MG63 osteoblastic-cell response by means of a microarray technology. J Periodontol. 2004;75:66–83. doi: 10.1902/jop.2004.75.1.66. [DOI] [PubMed] [Google Scholar]
- 406.Izzo MW, Pucci B, Tuan RS, Hall DJ. Gene expression profiling following BMP-2 induction of mesenchymal chondrogenesis in vitro. Osteoarthritis Cartilage. 2002;10:23–33. doi: 10.1053/joca.2001.0478. [DOI] [PubMed] [Google Scholar]
- 407.Clancy BM, Johnson JD, Lambert AJ, Rezvankhah S, Wong A, Resmini C, Feldman JL, Leppanen S, Pittman DD. A gene expression profile for endochondral bone formation: Oligonucleotide microarrays establish novel connections between known genes and BMP-2-induced bone formation in mouse quadriceps. Bone. 2003;33:46–63. doi: 10.1016/s8756-3282(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 408.Li V, Raouf A, Kitching R, Seth A. Ets2 transcription factor inhibits mineralization and affects target gene expression during osteoblast maturation. In Vivo. 2004;18:517–524. [PubMed] [Google Scholar]
- 409.Lotinun S, Sibonga JD, Turner RT. Differential effects of intermittent and continuous administration of parathyroid hormone on bone histomorphometry and gene expression. Endocrine. 2002;17:29–36. doi: 10.1385/ENDO:17:1:29. [DOI] [PubMed] [Google Scholar]
- 410.Nakazawa T, Nakajima A, Seki N, Okawa A, Kato M, Moriya H, Amizuka N, Einhorn TA, Yamazaki M. Gene expression of periostin in the early stage of fracture healing detected by cDNA microarray analysis. J Orthop Res. 2004;22:520–525. doi: 10.1016/j.orthres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 411.Nagel DE, Khosla S, Sanyal A, Rosen DM, Kumagai Y, Riggs BL. A fragment of the hypophosphatemic factor, MEPE, requires inducible cyclooxygenase-2 to exert potent anabolic effects on normal human marrow osteoblast precursors. J Cell Biochem. 2004;93:1107–1114. doi: 10.1002/jcb.20249. [DOI] [PubMed] [Google Scholar]
- 412.Stock M, Schafer H, Fliegauf M, Otto F. Identification of novel genes of the bone-specific transcription factor Runx2. J Bone Miner Res. 2004;19:959–972. doi: 10.1359/jbmr.2004.19.6.959. [DOI] [PubMed] [Google Scholar]
- 413.Hatano H, Siegel HJ, Yamagiwa H, Bronk JT, Turner RT, Bolander ME, Sarkar G. Identification of estrogen-regulated genes during fracture healing, using DNA microarray. J Bone Miner Metab. 2004;22:224–235. doi: 10.1007/s00774-003-0482-y. [DOI] [PubMed] [Google Scholar]
- 414.Segev O, Samach A, Faerman A, Kalinski H, Beiman M, Gelfand A, Turam H, Boguslavsky S, Moshayov A, Gottlieb H, Kazanov E, Nevo Z, Robinson D, Skaliter R, Einat P, Binderman I, Feinstein E. CMF608-a novel mechanical strain-induced bone-specific protein expressed in early osteochondroprogenitor cells. Bone. 2004;34:246–260. doi: 10.1016/j.bone.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 415.Meyer MH, Etienne W, Meyer RA., Jr Altered mRNA expression of genes related to nerve cell activity in the fracture callus of older rats: A randomized, controlled, microarray study. BMC Musculoskelet Disord. 2004;5:24. doi: 10.1186/1471-2474-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, Gerstenfeld LC, Einhorn TA. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33:889–898. doi: 10.1016/j.bone.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 417.Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem. 2002;277:30177–30182. doi: 10.1074/jbc.M203171200. [DOI] [PubMed] [Google Scholar]
- 418.Joo J, Ahn H, Lombardo F, Hadjiargyrou M, Zhu W. Statistical approaches in the analysis of gene expression data derived from bone regeneration specific cDNA microarrays. J Biopharm Stat. 2004;14:607–628. doi: 10.1081/BIP-200025652. [DOI] [PubMed] [Google Scholar]
- 419.Etienne W, Meyer MH, Peppers J, Meyer RA., Jr Comparison of mRNA gene expression by RT-PCR and DNA microarray. Biotechniques. 2004;36:618–626. doi: 10.2144/04364ST02. [DOI] [PubMed] [Google Scholar]
- 420.Farach-Carson MC, Xu Y. Microarray detection of gene expression changes induced by 1,25(OH)(2)D(3) and a Ca(2+) influx-activating analog in osteoblastic ROS 17/2.8 cells. Steroids. 2002;67:467–470. doi: 10.1016/s0039-128x(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 421.Fujita M, Urano T, Horie K, Ikeda K, Tsukui T, Fukuoka H, Tsutsumi O, Ouchi Y, Inoue S. Estrogen activates cyclin-dependent kinases 4 and 6 through induction of cyclin D in rat primary osteoblasts. Biochem Biophys Res Commun. 2002;299:222–228. doi: 10.1016/s0006-291x(02)02640-2. [DOI] [PubMed] [Google Scholar]
- 422.Harvey CB, Stevens DA, Williams AJ, Jackson DJ, O’Shea P, Williams GR. Analysis of thyroid hormone responsive gene expression in osteoblastic cells. Mol Cell Endocrinol. 2003;213:87–97. doi: 10.1016/j.mce.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 423.Qin L, Qiu P, Wang L, Li X, Swarthout JT, Soteropoulos P, Tolias P, Partridge NC. Gene expression profiles and transcription factors involved in parathyroid hormone signaling in osteoblasts revealed by microarray and bioinformatics. J Biol Chem. 2003;278:19723–19731. doi: 10.1074/jbc.M212226200. [DOI] [PubMed] [Google Scholar]


