Abstract
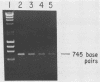
By using two highly conserved region of the luxA gene as primers, polymerase chain reaction amplification methods were used to prepare species-specific probes against the luciferase gene from four major groups of marine luminous bacteria. Laboratory studies with test strains indicated that three of the four probes cross-reacted with themselves and with one or more of the other species at low stringencies but were specific for members of their own species at high stringencies. The fourth probe, generated from Vibrio harveyi DNA, cross-reacted with DNAs from two closely related species, V. orientalis and V. vulnificus. When nonluminous cultures were tested with the species-specific probes, no false-positive results were observed, even at low stringencies. Two field isolates were correctly identified as Photobacterium phosphoreum by using the species-specific hybridization probes at high stringency. A mixed probe (four different hybridization probes) used at low stringency gave positive results with all of the luminous bacteria tested, including the terrestrial species, Xenorhabdus luminescens, and the taxonomically distinct marine bacterial species Shewanella hanedai; minimal cross-hybridization with these species was seen at higher stringencies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. O., Devine J. H., Heckel R. C., Lin J. W., Shadel G. S. The complete nucleotide sequence of the lux regulon of Vibrio fischeri and the luxABN region of Photobacterium leiognathi and the mechanism of control of bacterial bioluminescence. J Biolumin Chemilumin. 1989 Jul;4(1):326–341. doi: 10.1002/bio.1170040145. [DOI] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Woolkalis M. J., Bang S. S. Evolutionary relationships in vibrio and Photobacterium: a basis for a natural classification. Annu Rev Microbiol. 1983;37:369–398. doi: 10.1146/annurev.mi.37.100183.002101. [DOI] [PubMed] [Google Scholar]
- Boettcher K. J., Ruby E. G. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990 Jul;172(7):3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Mileham A. J., Simon M. I., Nealson K. H., Rausch S. K., Bonam D., Baldwin T. O. Nucleotide sequence of the luxA gene of Vibrio harveyi and the complete amino acid sequence of the alpha subunit of bacterial luciferase. J Biol Chem. 1985 May 25;260(10):6139–6146. [PubMed] [Google Scholar]
- Cohn D. H., Ogden R. C., Abelson J. N., Baldwin T. O., Nealson K. H., Simon M. I., Mileham A. J. Cloning of the Vibrio harveyi luciferase genes: use of a synthetic oligonucleotide probe. Proc Natl Acad Sci U S A. 1983 Jan;80(1):120–123. doi: 10.1073/pnas.80.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. W., Sizemore R. K. Incidence of Vibrio species associated with blue crabs (Callinectes sapidus) collected from Galveston Bay, Texas. Appl Environ Microbiol. 1982 May;43(5):1092–1097. doi: 10.1128/aem.43.5.1092-1097.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Foran D. R., Brown W. M. Nucleotide sequence of the LuxA and LuxB genes of the bioluminescent marine bacterium Vibrio fischeri. Nucleic Acids Res. 1988 Jan 25;16(2):777–777. doi: 10.1093/nar/16.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W. Biological diversity, chemical mechanisms, and the evolutionary origins of bioluminescent systems. J Mol Evol. 1983;19(5):309–321. doi: 10.1007/BF02101634. [DOI] [PubMed] [Google Scholar]
- Haygood M. G., Cohn D. H. Luciferase genes cloned from the unculturable luminous bacteroid symbiont of the Caribbean flashlight fish, Kryptophanaron alfredi. Gene. 1986;45(2):203–209. doi: 10.1016/0378-1119(86)90255-6. [DOI] [PubMed] [Google Scholar]
- Johnston T. C., Rucker E. B., Cochrum L., Hruska K. S., Vandegrift V. The nucleotide sequence of the luxA and luxB genes of Xenorhabdus luminescens HM and a comparison of the amino acid sequences of luciferases from four species of bioluminescent bacteria. Biochem Biophys Res Commun. 1990 Jul 31;170(2):407–415. doi: 10.1016/0006-291x(90)92106-a. [DOI] [PubMed] [Google Scholar]
- Leisman G., Cohn D. H., Nealson K. H. Bacterial origin of luminescence in marine animals. Science. 1980 Jun 13;208(4449):1271–1273. doi: 10.1126/science.208.4449.1271. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988 Jun 3;240(4857):1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- Nealson K. H., Hastings J. W. Bacterial bioluminescence: its control and ecological significance. Microbiol Rev. 1979 Dec;43(4):496–518. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'brien C. H., Sizemore R. K. Distribution of the Luminous Bacterium Beneckea harveyi in a Semitropical Estuarine Environment. Appl Environ Microbiol. 1979 Nov;38(5):928–933. doi: 10.1128/aem.38.5.928-933.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Roberts D. M., White V. K., Dry M. A., Simpson L. M. Bioluminescence in a strain of the human pathogenic bacterium Vibrio vulnificus. Appl Environ Microbiol. 1986 Nov;52(5):1209–1211. doi: 10.1128/aem.52.5.1209-1211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff S. A., Colwell R. R. Distribution and identification of luminous bacteria from the sargasso sea. Appl Environ Microbiol. 1980 May;39(5):983–987. doi: 10.1128/aem.39.5.983-987.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Greenberg E. P., Hastings J. W. Planktonic marine luminous bacteria: species distribution in the water column. Appl Environ Microbiol. 1980 Feb;39(2):302–306. doi: 10.1128/aem.39.2.302-306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Morin J. G. Luminous enteric bacteria of marine fishes: a study of their distribution, densities, and dispersion. Appl Environ Microbiol. 1979 Sep;38(3):406–411. doi: 10.1128/aem.38.3.406-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica; a model of symbiosis based on bacterial studies. Biol Bull. 1976 Dec;151(3):574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shilo M., Yetinson T. Physiological characteristics underlying the distribution patterns of luminous bacteria in the mediterranean sea and the gulf of elat. Appl Environ Microbiol. 1979 Oct;38(4):577–584. doi: 10.1128/aem.38.4.577-584.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West P. A., Lee J. V., Bryant T. N. A numerical taxonomic study of species of Vibrio isolated from the aquatic environment and birds in Kent, England. J Appl Bacteriol. 1983 Oct;55(2):263–282. doi: 10.1111/j.1365-2672.1983.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Yetinson T., Shilo M. Seasonal and geographic distribution of luminous bacteria in the eastern mediterranean sea and the gulf of elat. Appl Environ Microbiol. 1979 Jun;37(6):1230–1238. doi: 10.1128/aem.37.6.1230-1238.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]