Abstract
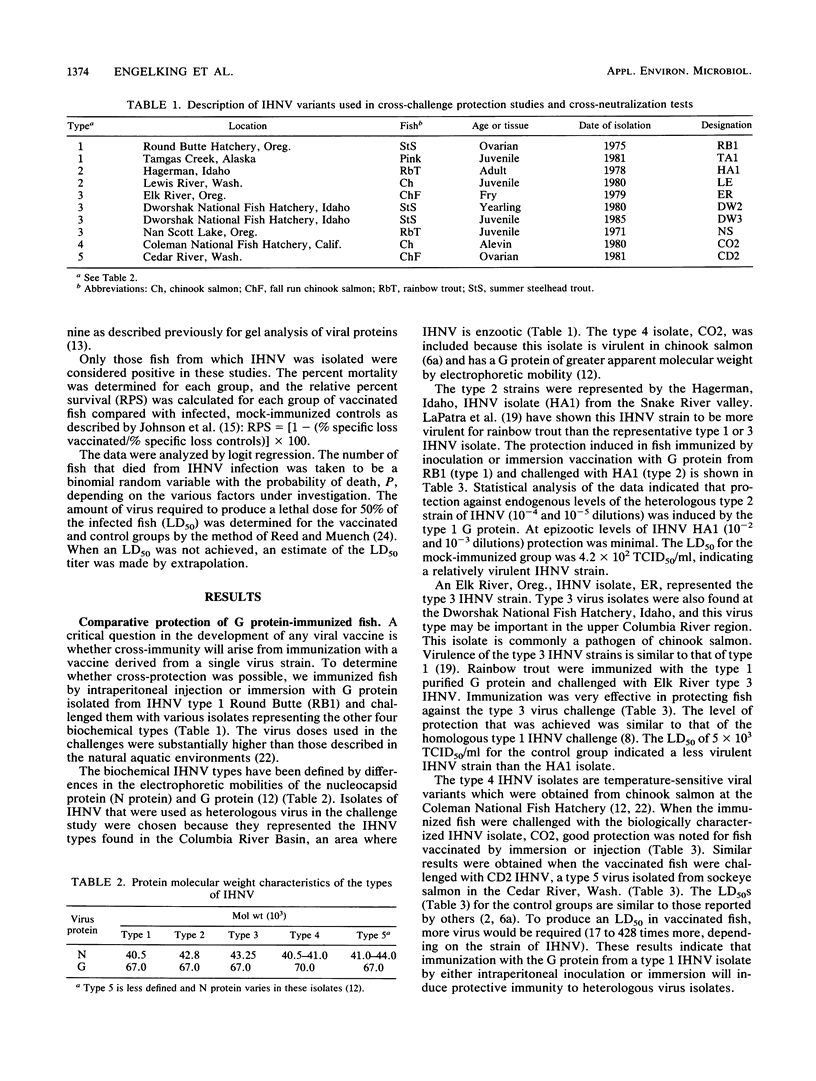
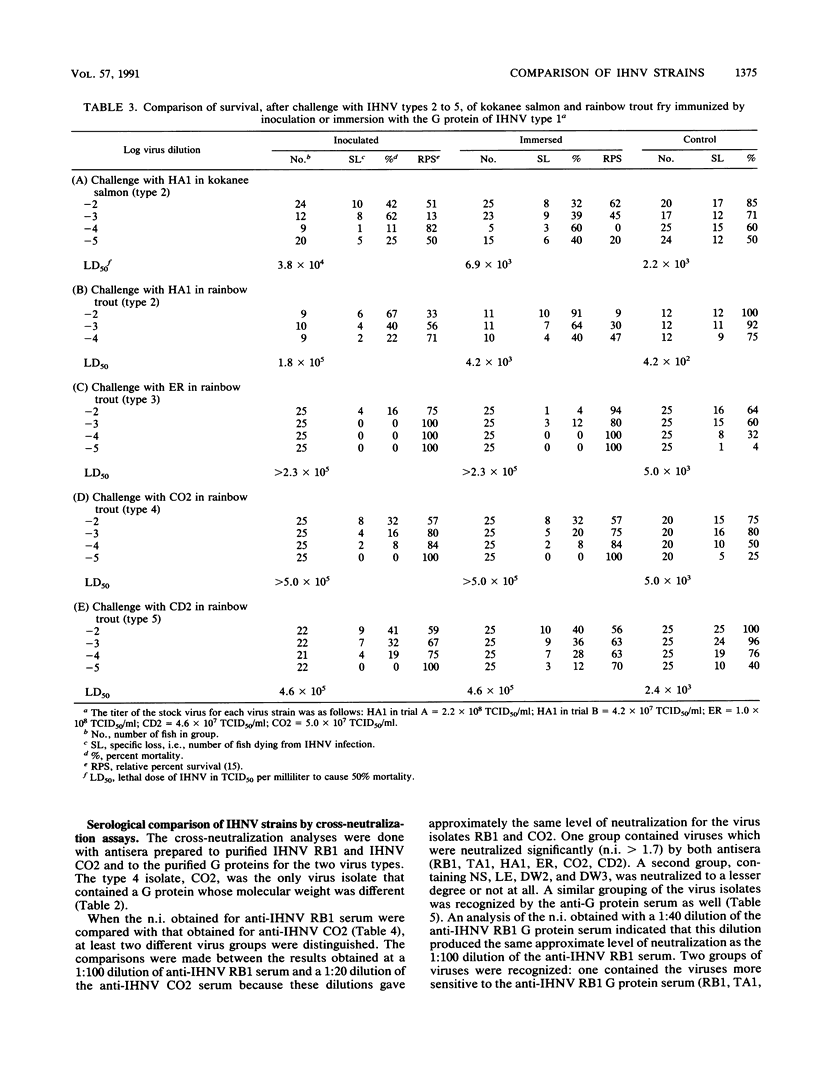
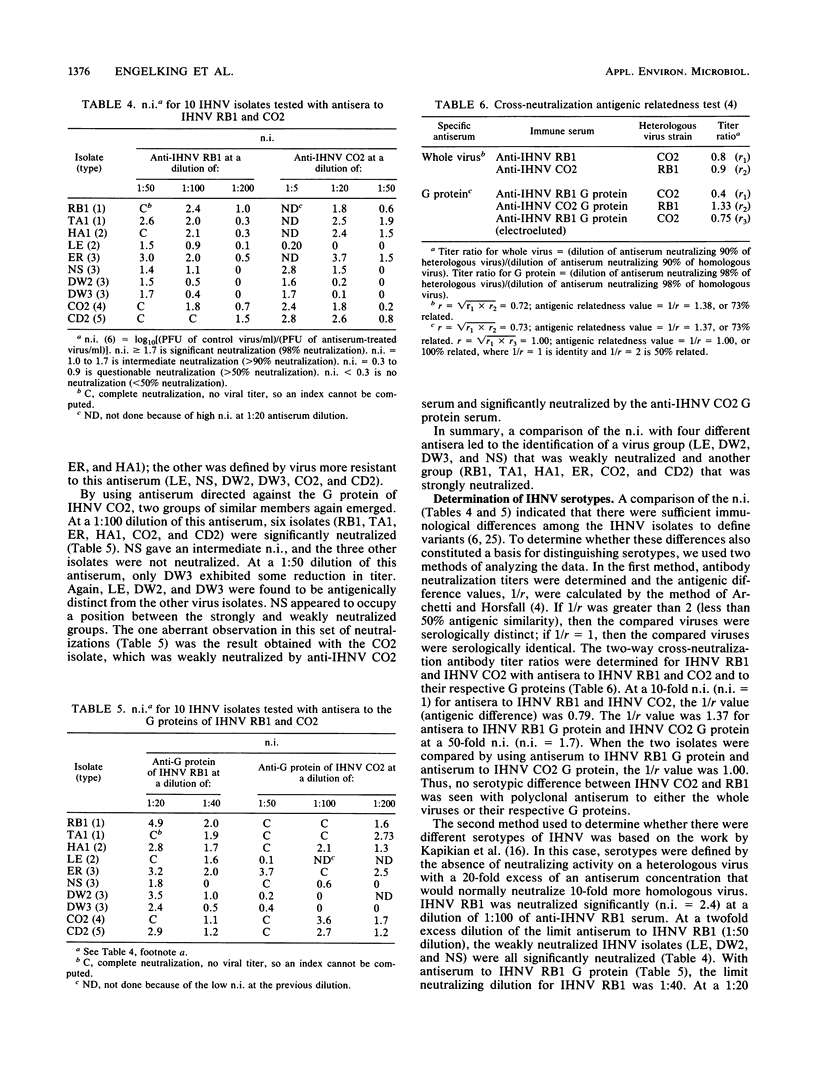
Infectious hematopoietic necrosis virus (IHNV) is a pathogen of young salmon and trout. Viral epizootics among these fish in private and public rearing facilities have been a problem in the northwestern United States from California to Alaska, and an IHNV vaccine has been sought by the aquaculture experts. Since an IHNV vaccine must be designed to immunize against all viral serotypes, an analysis of IHNV serotypes was made. A large number of viruses from widely separated geographic locations and different fish species had already been placed in one of five electropherotypes by the migration of the virion proteins in sodium dodecyl sulfate-polyacrylamide gels. Also, there was evidence that some of these virus isolates had differences in virulence for chinook salmon, rainbow trout, or kokanee salmon. Previous serological studies with polyclonal rabbit antisera and three IHNV isolates indicated that there was only one serotype (B. B. McCain, J. L. Fryer, and K. S. Pilcher, Proc. Soc. Exp. Biol. Med. 137:1042-1046, 1971). A substantial number of new IHNV isolations have been made since that study, and thus a more extensive comparison was made of 10 different IHNV isolates representing the five electropherotypes. This report shows that the glycoprotein from a single isolate of IHNV can induce a protective immune response in vivo to the five IHNV electropherotypes. Plaque reduction neutralization assays indicated that there was only one serotype. Thus, despite the differences observed in the migration of the structural proteins for IHNV isolated from separate geographic locations and different fish species, only one neutralizing virus type was identified.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHETTI I., HORSFALL F. L., Jr Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J Exp Med. 1950 Nov 1;92(5):441–462. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. A., Mulcahy D. Plaquing procedure for infectious hematopoietic necrosis virus. Appl Environ Microbiol. 1980 Apr;39(4):872–876. doi: 10.1128/aem.39.4.872-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. H., Dietzschold B., Schneider L. G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977 Jun;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelking H. M., Leong J. C. IHNV persistently infects chinook salmon embryo cells. Virology. 1981 Feb;109(1):47–58. doi: 10.1016/0042-6822(81)90470-0. [DOI] [PubMed] [Google Scholar]
- Engelking H. M., Leong J. C. The glycoprotein of infectious hematopoietic necrosis virus elicits neutralizing antibody and protective responses. Virus Res. 1989 Jul;13(3):213–230. doi: 10.1016/0168-1702(89)90017-8. [DOI] [PubMed] [Google Scholar]
- Hsu Y. L., Engelking H. M., Leong J. C. Occurrence of different types of infectious hematopoietic necrosis virus in fish. Appl Environ Microbiol. 1986 Dec;52(6):1353–1361. doi: 10.1128/aem.52.6.1353-1361.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Virion RNA polymerases of two salmonid rhabdoviruses. J Virol. 1977 Jun;22(3):839–843. doi: 10.1128/jvi.22.3.839-843.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain B. B., Fryer J. L., Pilcher K. S. Antigenic relationships in a group of three viruses of salmonid fish by cross neutralization. Proc Soc Exp Biol Med. 1971 Jul;137(3):1042–1046. doi: 10.3181/00379727-137-35724. [DOI] [PubMed] [Google Scholar]
- Pilcher K. S., Fryer J. L. The viral diseases of fish: a review through 1978. Part 1: Diseases of proven viral etiology. Crit Rev Microbiol. 1980;7(4):287–363. doi: 10.3109/10408418009077984. [DOI] [PubMed] [Google Scholar]
- Rhinoviruses: a numbering system. Nature. 1967 Feb 25;213(5078):761–762. doi: 10.1038/213761a0. [DOI] [PubMed] [Google Scholar]
- Schneider L. G., Dietzschold B., Dierks R. E., Matthaeus W., Enzmann P. J., Strohmaier K. Rabies group-specific ribonucleoprotein antigen and a test system for grouping and typing of rhabdoviruses. J Virol. 1973 May;11(5):748–755. doi: 10.1128/jvi.11.5.748-755.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Reagan K. J., Dietzschold B., Curtis P. J., Wunner W. H., Kieny M. P., Lathe R., Lecocq J. P., Mackett M. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]


