Abstract
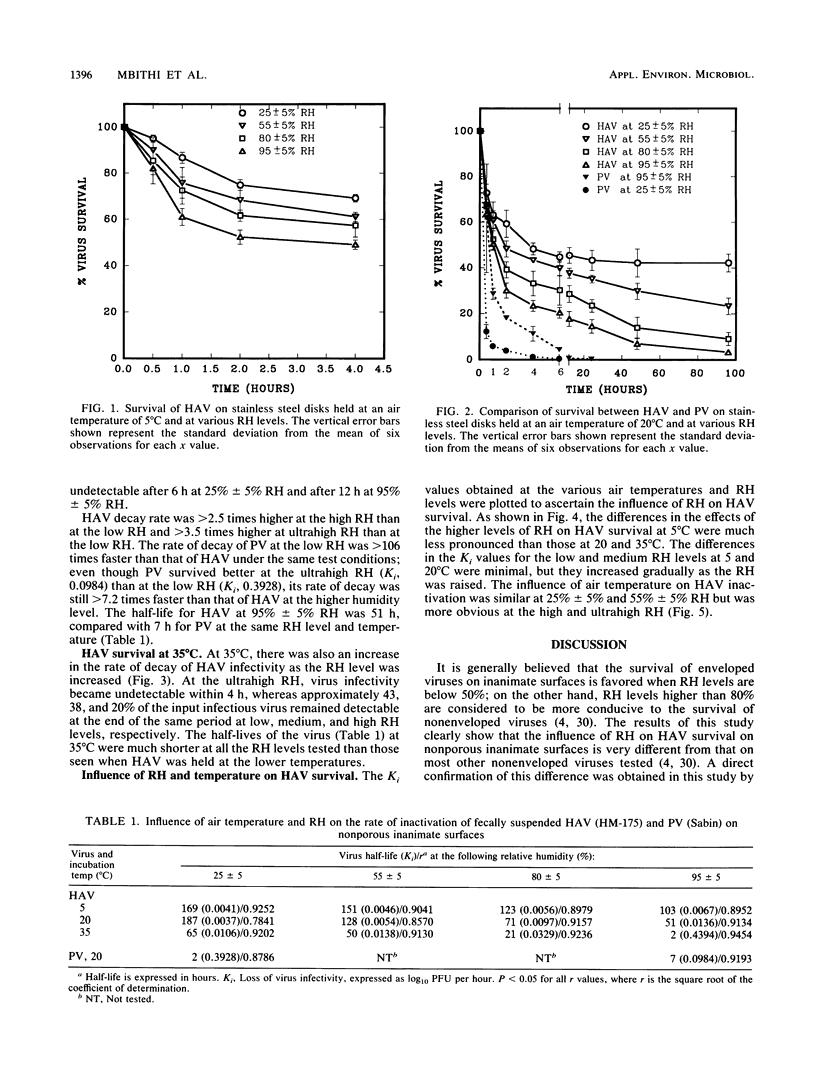
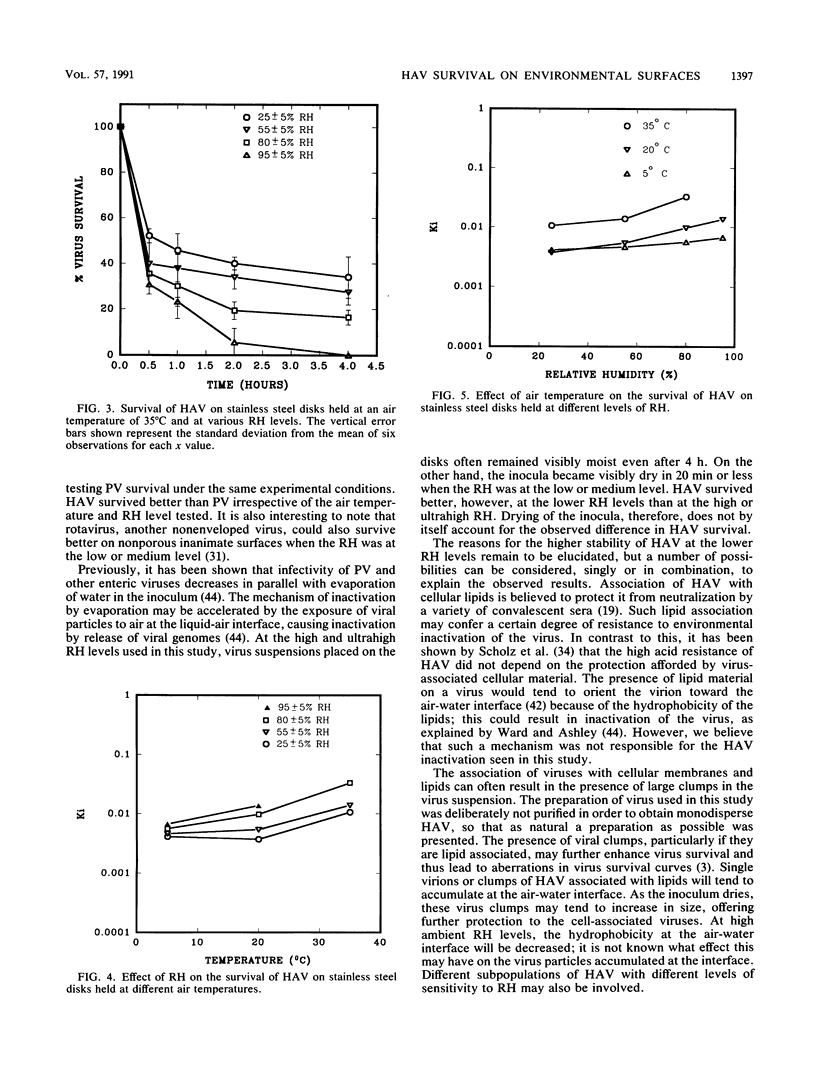
Stainless steel disks (diameter, 1 cm) were contaminated with fecally suspended hepatitis A virus (HAV; strain HM-175) and held at low (25% +/- 5%), medium (55% +/- 5%), high (80% +/- 5%), or ultrahigh (95% +/- 5%) relative humidity (RH) at an air temperature of 5,20, or 35 degrees C. HAV survival was inversely proportional to the level of RH and temperature, and the half-lives of the virus ranged from greater than 7 days at the low RH and 5 degrees C to about 2 h at the ultrahigh RH and 35 degrees C. In parallel tests with fecally suspended Sabin poliovirus (PV) type 1 at the low and ultrahigh RH, all PV activity was lost within 4 h at the low RH whereas at the ultrahigh RH it remained detectable up to 12 h. HAV could therefore survive much better than PV on nonporous environmental surfaces. Moreover, the ability of HAV to survive better at low levels of RH is in direct contrast to the behavior of other enteroviruses. These findings should help in understanding the genesis of HAV outbreaks more clearly and in designing better measures for their control and prevention.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aach R. D., Evans J., Losee J. An epidemic of infectious hepatitis possibly due to airborne transmission. Am J Epidemiol. 1968 Jan;87(1):99–109. doi: 10.1093/oxfordjournals.aje.a120813. [DOI] [PubMed] [Google Scholar]
- Ansari S. A., Sattar S. A., Springthorpe V. S., Wells G. A., Tostowaryk W. Rotavirus survival on human hands and transfer of infectious virus to animate and nonporous inanimate surfaces. J Clin Microbiol. 1988 Aug;26(8):1513–1518. doi: 10.1128/jcm.26.8.1513-1518.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKLAND F. E., TYRRELL D. A. Loss of infectivity on drying various viruses. Nature. 1962 Sep 15;195:1063–1064. doi: 10.1038/1951063a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulepis A. G., Anderson B. N., Gust I. D. Hepatitis A. Adv Virus Res. 1987;32:129–169. doi: 10.1016/s0065-3527(08)60476-5. [DOI] [PubMed] [Google Scholar]
- ENRIGHT J. R. The epidemiology of paralytic poliomyelitis in Hawaii. Hawaii Med J. 1954 May-Jun;13(5):350–354. [PubMed] [Google Scholar]
- Gingrich G. A., Hadler S. C., Elder H. A., Ash K. O. Serologic investigation of an outbreak of hepatitis A in a rural day-care center. Am J Public Health. 1983 Oct;73(10):1190–1193. doi: 10.2105/ajph.73.10.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R. A., Carder C. C., Allen J. R., Orenstein W. A., Finton R. J. Nosocomial hepatitis A transmission by an adult patient with diarrhea. Am J Med. 1982 Aug;73(2):220–226. doi: 10.1016/0002-9343(82)90182-6. [DOI] [PubMed] [Google Scholar]
- HEMMES J. H., WINKLER K. C., KOOL S. M. Virus survival as a seasonal factor in influenza and polimyelitis. Nature. 1960 Oct 29;188:430–431. doi: 10.1038/188430a0. [DOI] [PubMed] [Google Scholar]
- Hadler S. C., McFarland L. Hepatitis in day care centers: epidemiology and prevention. Rev Infect Dis. 1986 Jul-Aug;8(4):548–557. doi: 10.1093/clinids/8.4.548. [DOI] [PubMed] [Google Scholar]
- Hadler S. C., Webster H. M., Erben J. J., Swanson J. E., Maynard J. E. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med. 1980 May 29;302(22):1222–1227. doi: 10.1056/NEJM198005293022203. [DOI] [PubMed] [Google Scholar]
- Klein B. S., Michaels J. A., Rytel M. W., Berg K. G., Davis J. P. Nosocomial hepatitis A. A multinursery outbreak in Wisconsin. JAMA. 1984 Nov 16;252(19):2716–2721. doi: 10.1001/jama.252.19.2716. [DOI] [PubMed] [Google Scholar]
- Lehmann N. I., Sharma D. L., Gust I. D. Prevalence of antibody to the hepatitis A virus in a large institution for the mentally retarded. J Med Virol. 1978;2(4):335–339. doi: 10.1002/jmv.1890020406. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N. Antigenic relatedness of two strains of hepatitis A virus determined by cross-neutralization. Infect Immun. 1983 Oct;42(1):418–420. doi: 10.1128/iai.42.1.418-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. S., Fontaine R. E., Smith C. A., Brinda J., Hirman G., Nelson D. B., Johnson P. M., Larson O. A large food-borne outbreak of hepatitis A. Possible transmission via oropharyngeal secretions. JAMA. 1975 Oct 20;234(3):289–294. [PubMed] [Google Scholar]
- Matthew E. B., Dietzman D. E., Madden D. L., Newman S. J., Sever J. L., Nagler B., Bouton S. M., Rostafinski M. A major epidmeic of infectious hepatitis in an institution for the mentally retarded. Am J Epidemiol. 1973 Sep;98(3):199–215. doi: 10.1093/oxfordjournals.aje.a121549. [DOI] [PubMed] [Google Scholar]
- Mbithi J. N., Springthorpe V. S., Sattar S. A. Chemical disinfection of hepatitis A virus on environmental surfaces. Appl Environ Microbiol. 1990 Nov;56(11):3601–3604. doi: 10.1128/aem.56.11.3601-3604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaustland K. A., Bond W. W., Bradley D. W., Ebert J. W., Maynard J. E. Survival of hepatitis A virus in feces after drying and storage for 1 month. J Clin Microbiol. 1982 Nov;16(5):957–958. doi: 10.1128/jcm.16.5.957-958.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L. Classification of hepatitis A virus as enterovirus type 72 and of hepatitis B virus as hepadnavirus type 1. Intervirology. 1982;18(3):105–106. doi: 10.1159/000149313. [DOI] [PubMed] [Google Scholar]
- Moore M. Centers for Disease Control. Enteroviral disease in the United States, 1970-1979. J Infect Dis. 1982 Jul;146(1):103–108. doi: 10.1093/infdis/146.1.103. [DOI] [PubMed] [Google Scholar]
- Naus M., Everett W., Davies S., Coutts J. A school outbreak of hepatitis A in southwestern Ontario. Can Dis Wkly Rep. 1989 Nov 11;15(45):225–228. [PubMed] [Google Scholar]
- Petersen N. J., Bressler G. K. Design and modification of the day care environment. Rev Infect Dis. 1986 Jul-Aug;8(4):618–621. doi: 10.1093/clinids/8.4.618. [DOI] [PubMed] [Google Scholar]
- Polakoff S. Reports of clinical hepatitis A from Public Health and hospital microbiology laboratories to the PHLS Communicable Disease Surveillance Centre during the period 1980-1988. J Infect. 1990 Jul;21(1):111–117. doi: 10.1016/0163-4453(90)90853-z. [DOI] [PubMed] [Google Scholar]
- Ramia S., Sattar S. A. Second-step concentration of viruses in drinking and surface waters using polyethylene glycol hydroextraction. Can J Microbiol. 1979 May;25(5):587–592. doi: 10.1139/m79-084. [DOI] [PubMed] [Google Scholar]
- Sattar S. A., Dimock K. D., Ansari S. A., Springthorpe V. S. Spread of acute hemorrhagic conjunctivitis due to enterovirus-70: effect of air temperature and relative humidity on virus survival on fomites. J Med Virol. 1988 Jul;25(3):289–296. doi: 10.1002/jmv.1890250306. [DOI] [PubMed] [Google Scholar]
- Sattar S. A., Lloyd-Evans N., Springthorpe V. S., Nair R. C. Institutional outbreaks of rotavirus diarrhoea: potential role of fomites and environmental surfaces as vehicles for virus transmission. J Hyg (Lond) 1986 Apr;96(2):277–289. doi: 10.1017/s0022172400066055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar S. A., Springthorpe V. S., Karim Y., Loro P. Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiol Infect. 1989 Jun;102(3):493–505. doi: 10.1017/s0950268800030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon J. W., Leikkanen M. The use of fluorescein powder for evaluating contamination in a newborn nursery. J Pediatr. 1973 Jun;82(6):966–971. doi: 10.1016/s0022-3476(73)80426-3. [DOI] [PubMed] [Google Scholar]
- Scholz E., Heinricy U., Flehmig B. Acid stability of hepatitis A virus. J Gen Virol. 1989 Sep;70(Pt 9):2481–2485. doi: 10.1099/0022-1317-70-9-2481. [DOI] [PubMed] [Google Scholar]
- Siegl G., Lemon S. M. Recent advances in hepatitis A vaccine development. Virus Res. 1990 Oct;17(2):75–92. doi: 10.1016/0168-1702(90)90070-r. [DOI] [PubMed] [Google Scholar]
- Siegl G., Weitz M., Kronauer G. Stability of hepatitis A virus. Intervirology. 1984;22(4):218–226. doi: 10.1159/000149554. [DOI] [PubMed] [Google Scholar]
- Smith M. S., Swanepoel P. J., Bootsma M. Hepatitis A in non-human primates in nature. Lancet. 1980 Dec 6;2(8206):1241–1242. doi: 10.1016/s0140-6736(80)92495-2. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Oglesbee S. E., Wait D. A. Evaluation of methods for concentrating hepatitis A virus from drinking water. Appl Environ Microbiol. 1985 Dec;50(6):1457–1463. doi: 10.1128/aem.50.6.1457-1463.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticehurst J. R. Hepatitis A virus: clones, cultures, and vaccines. Semin Liver Dis. 1986 Feb;6(1):46–55. doi: 10.1055/s-2008-1040793. [DOI] [PubMed] [Google Scholar]
- Trouwborst T., Kuyper S., de Jong J. C., Plantinga A. D. Inactivation of some bacterial and animal viruses by exposure to liquid-air interfaces. J Gen Virol. 1974 Jul;24(1):155–165. doi: 10.1099/0022-1317-24-1-155. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Inactivation of enteric viruses in wastewater sludge through dewatering by evaporation. Appl Environ Microbiol. 1977 Nov;34(5):564–570. doi: 10.1128/aem.34.5.564-570.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef G. E., Brown I. N., Mowbray J. F. Derivation and biochemical characterization of an enterovirus group-specific monoclonal antibody. Intervirology. 1987;28(3):163–170. doi: 10.1159/000150012. [DOI] [PubMed] [Google Scholar]



