Abstract
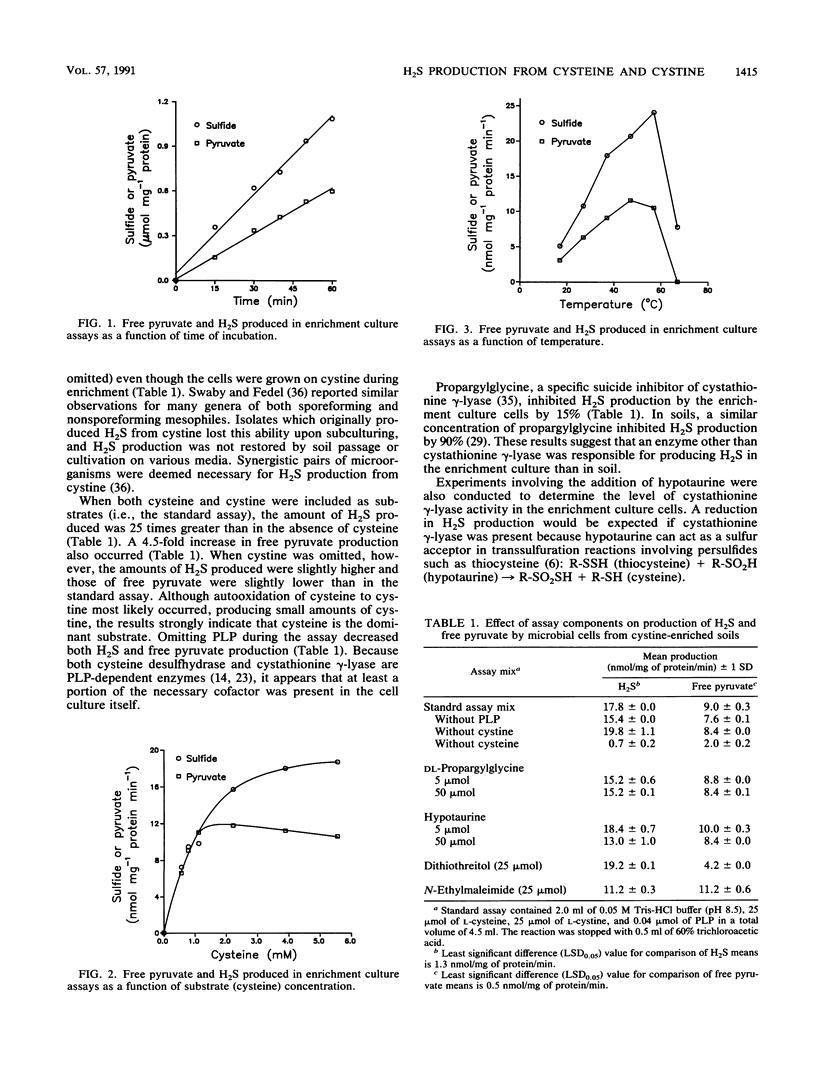
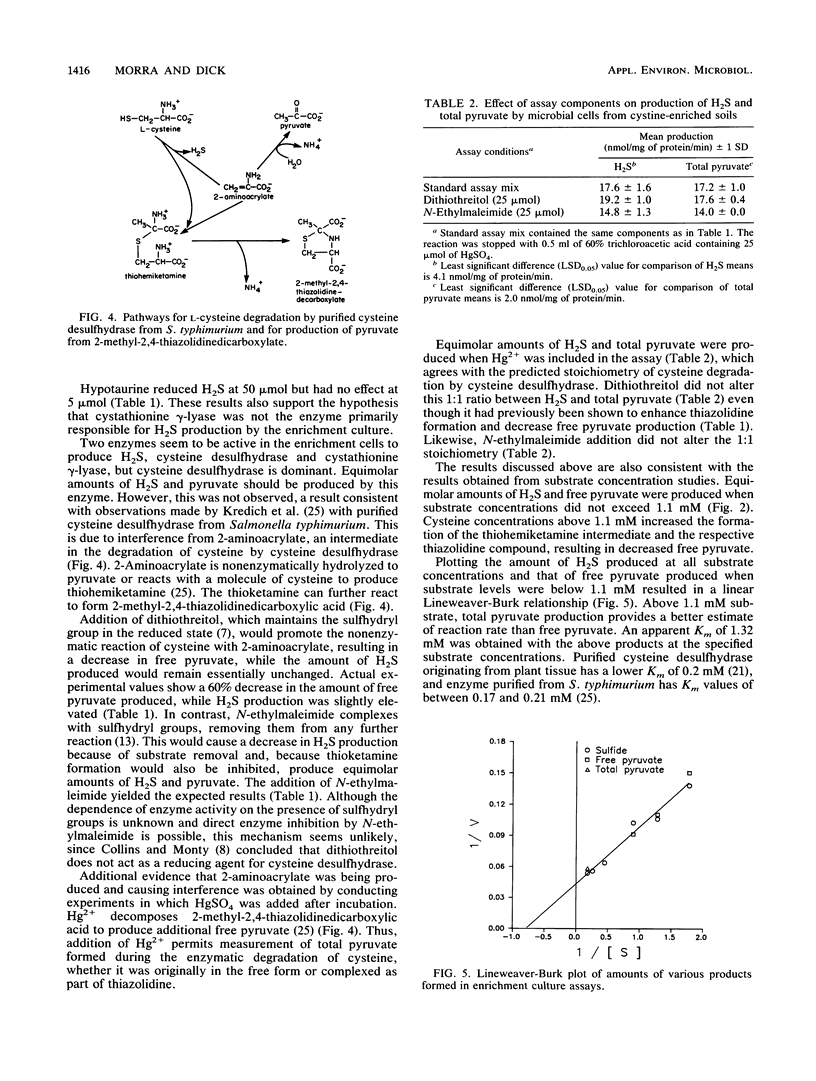
Hydrogen sulfide (H2S) is a major component of biogenic gaseous sulfur emissions from terrestrial environments. However, little is known concerning the pathways for H2S production from the likely substrates, cysteine and cystine. A mixed microbial culture obtained from cystine-enriched soils was used in assays (50 min, 37°C) with 0.05 M Tris-HCl (pH 8.5), 25 μmol of l-cysteine, 25 μmol of l-cystine, and 0.04 μmol of pyridoxal 5′-phosphate. Sulfide was trapped in a center well containing zinc acetate, while pyruvate was measured by derivatization with 2,4-dinitrophenylhydrazine. Sulfide and total pyruvate production were 17.6 and 17.2 nmol mg of protein-1 min-1, respectively. Dithiothreitol did not alter reaction stoichiometry or the amount of H2S and total pyruvate, whereas N-ethylmaleimide reduced both H2S and total pyruvate production equally. The amount of H2S produced was reduced by 96% when only l-cystine was included as the substrate in the assay and by 15% with the addition of propargylglycine, a specific suicide inhibitor of cystathionine γ-lyase. These data indicate that the substrate for the reaction was cysteine and the enzyme responsible for H2S and pyruvate production was cysteine desulfhydrase (EC 4.4.1.1). The enzyme had a Km of 1.32 mM and was inactivated by temperatures greater than 60°C. Because cysteine is present in soil and cysteine desulfhydrase is an inducible enzyme, the potential for H2S production by this mechanism exists in terrestrial environments. The relative importance of this mechanism compared with other processes involved in H2S production from soil is unknown.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAVALLINI D., MONDOVI B., DE MARCO C., SCIOSCIA-SANTORO A. The mechanism of desulphhydration of cysteine. Enzymologia. 1962 Jun 30;24:253–266. [PubMed] [Google Scholar]
- CAVALLINI D., MONDOVI B., DE MARCO C., SCIOSCIASANTORO A. Inhibitory effect of mercaptoethanol and hypotaurine on the desulfhydration of cysteine by cystathionase. Arch Biochem Biophys. 1962 Feb;96:456–457. doi: 10.1016/0003-9861(62)90436-8. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Collins J. M., Monty K. J. The cysteine desulfhydrase of Salmonella typhimurium. Kinetic and catalytic properties. J Biol Chem. 1973 Sep 10;248(17):5943–5949. [PubMed] [Google Scholar]
- DELAVIER-KLUTCHKO C., FLAVIN M. ENZYMATIC SYNTHESIS AND CLEAVAGE OF CYSTATHIONINE IN FUNGI AND BACTERIA. J Biol Chem. 1965 Jun;240:2537–2549. [PubMed] [Google Scholar]
- Ellis R. J. Sulphur metabolism: the usefulness of N-ethylmaleimide. Nature. 1966 Sep 17;211(5055):1266–1268. doi: 10.1038/2111266a0. [DOI] [PubMed] [Google Scholar]
- FLAVIN M. Microbial transsulfuration: the mechanism of an enzymatic disulfide elimination reaction. J Biol Chem. 1962 Mar;237:768–777. [PubMed] [Google Scholar]
- FLAVIN M., SEGAL A. PURIFICATION AND PROPERTIES OF THE CYSTATHIONINE GAMMA-CLEAVAGE ENZYME OF NEUROSPORA. J Biol Chem. 1964 Jul;239:2220–2227. [PubMed] [Google Scholar]
- Guarneros G., Ortega M. V. Cysteine desulfhydrase activities of Salmonella typhimurium and Escherichia coli. Biochim Biophys Acta. 1970 Jan 14;198(1):132–142. doi: 10.1016/0005-2744(70)90041-0. [DOI] [PubMed] [Google Scholar]
- Hargrove J. L. Persulfide generated from L-cysteine inactivates tyrosine aminotransferase. Requirement for a protein with cysteine oxidase activity and gamma-cystathionase. J Biol Chem. 1988 Nov 25;263(33):17262–17269. [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine metabolism in cultured tobacco cells. Plant Physiol. 1980 Jan;65(1):151–155. doi: 10.1104/pp.65.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLIO R. E. Function of pyridoxal phosphate in desulfhydrase systems of Proteus morganii. J Biol Chem. 1951 Sep;192(1):371–377. [PubMed] [Google Scholar]
- Kredich N. M., Foote L. J., Keenan B. S. The stoichiometry and kinetics of the inducible cysteine desulfhydrase from Salmonella typhimurium. J Biol Chem. 1973 Sep 10;248(17):6187–6196. [PubMed] [Google Scholar]
- Nagasawa T., Kanzaki H., Yamada H. Cystathionine gamma-lyase of Streptomyces phaeochromogenes. The occurrence of cystathionine gamma-lyase in filamentous bacteria and its purification and characterization. J Biol Chem. 1984 Aug 25;259(16):10393–10403. [PubMed] [Google Scholar]
- Stipanuk M. H., Beck P. W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982 Aug 15;206(2):267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Billy G., Muller P., Chatagner F. New insights into the active center of rat liver cystathionase. Biochim Biophys Acta. 1975 Jul 27;397(1):231–243. doi: 10.1016/0005-2744(75)90196-5. [DOI] [PubMed] [Google Scholar]



