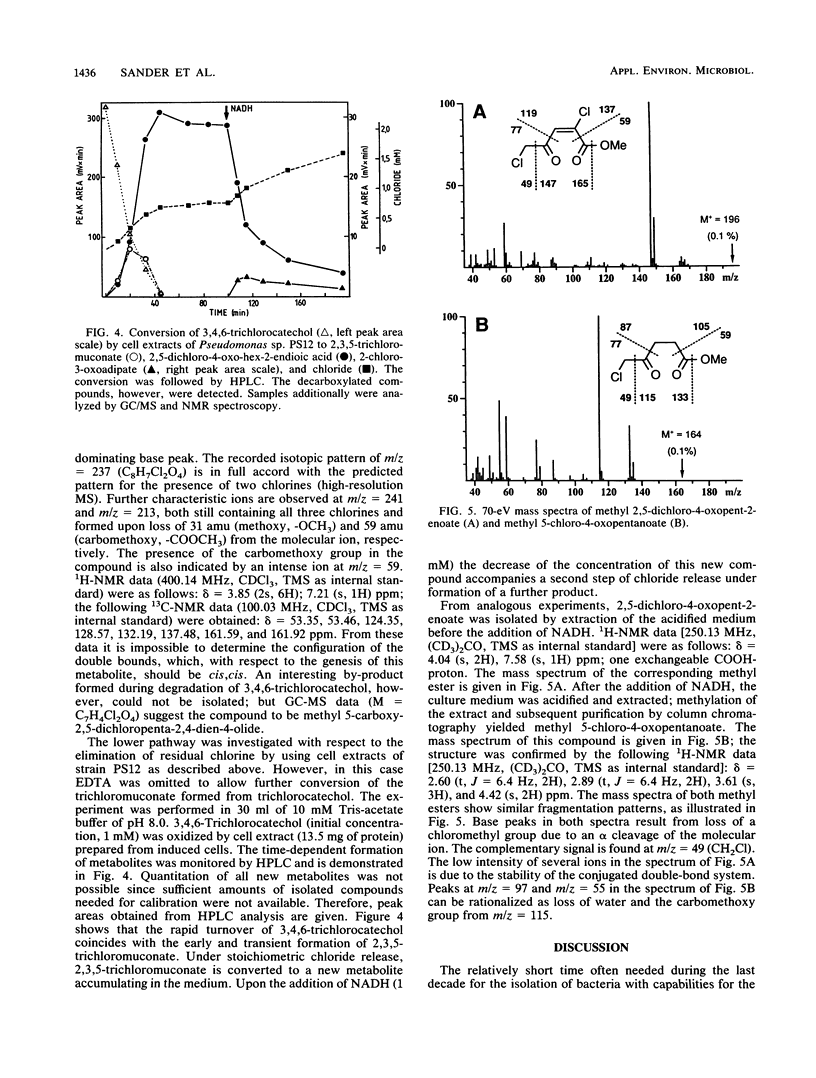
Abstract
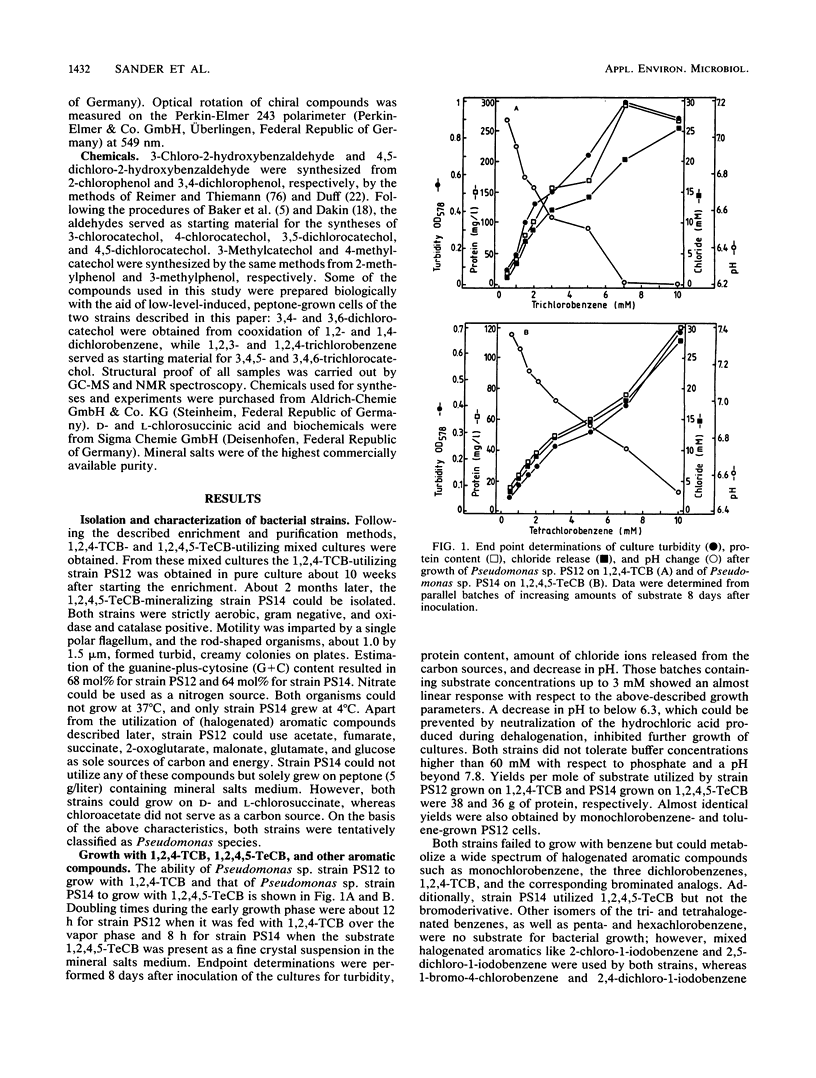
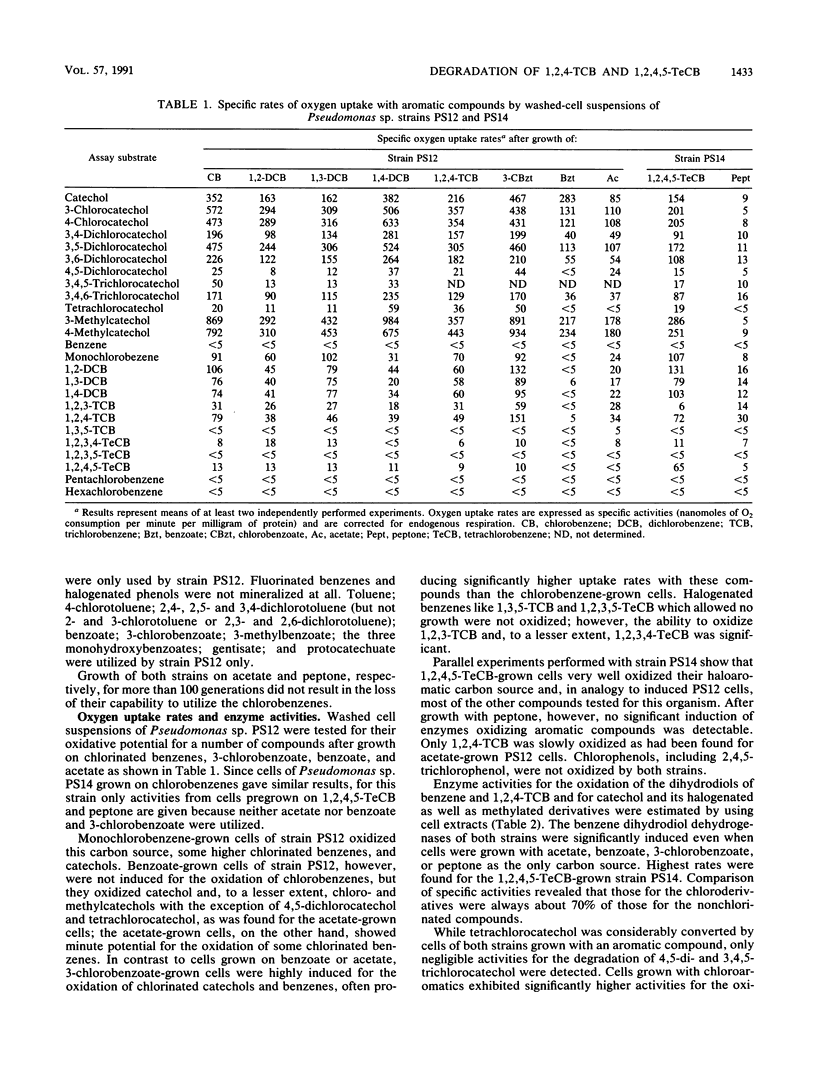
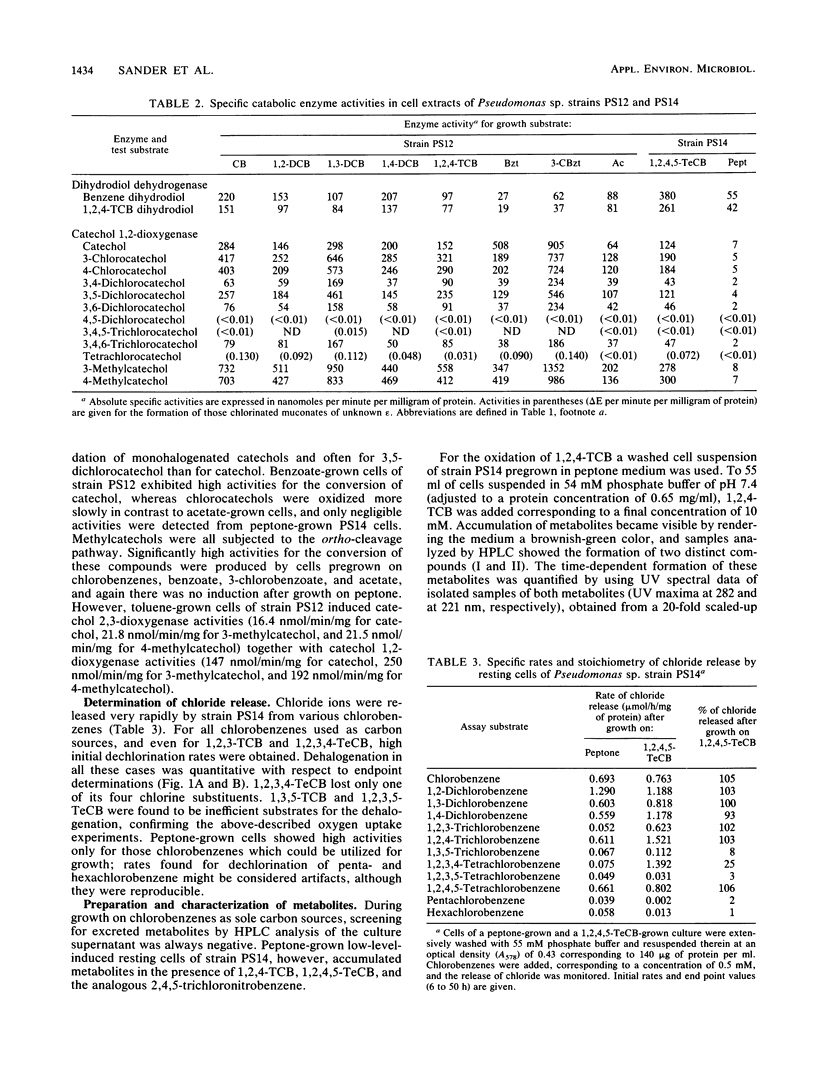
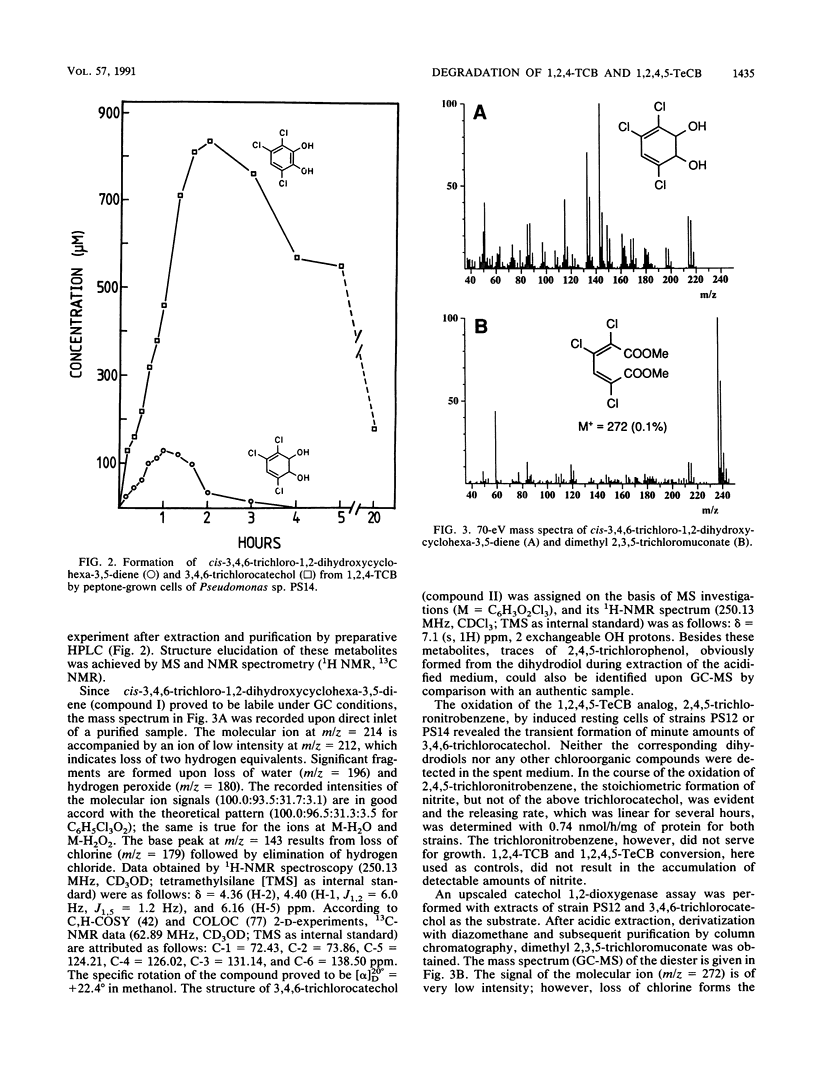
Two Pseudomonas sp. strains, capable of growth on chlorinated benzenes as the sole source of carbon and energy, were isolated by selective enrichment from soil samples of an industrial waste deposit. Strain PS12 grew on monochlorobenzene, all three isomeric dichlorobenzenes, and 1,2,4-trichlorobenzene (1,2,4-TCB). Strain PS14 additionally used 1,2,4,5-tetrachlorobenzene (1,2,4,5-TeCB). During growth on these compounds both strains released stoichiometric amounts of chloride ions. The first steps of the catabolism of 1,2,4-TCB and 1,2,4,5-TeCB proceeded via dioxygenation of the aromatic nuclei and furnished 3,4,6-trichlorocatechol. The intermediary cis-3,4,6-trichloro-1,2-dihydroxycyclohexa-3,5-diene (TCB dihydrodiol) formed from 1,2,4-TCB was rearomatized by an NAD+-dependent dihydrodiol dehydrogenase activity, while in the case of 1,2,4,5-TeCB oxidation the catechol was obviously produced by spontaneous elimination of hydrogen chloride from the initially formed 1,3,4,6-tetrachloro-1,2-dihydroxycyclohexa-3,5-diene. Subsequent ortho cleavage was catalyzed by a type II catechol 1,2-dioxygenase producing the corresponding 2,3,5-trichloromuconate which was channeled into the tricarboxylic acid pathway via an ordinary degradation sequence, which in the present case included 2-chloro-3-oxoadipate. From the structure-related compound 2,4,5-trichloronitrobenzene the nitro group was released as nitrite, leaving the above metabolite as 3,4,6-trichlorocatechol. Enzyme activities for the oxidation of chlorobenzenes and halogenated metabolites were induced by both strains during growth on these haloaromatics and, to a considerable extent, during growth of strain PS12 on acetate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aelion C. M., Swindoll C. M., Pfaender F. K. Adaptation to and biodegradation of xenobiotic compounds by microbial communities from a pristine aquifer. Appl Environ Microbiol. 1987 Sep;53(9):2212–2217. doi: 10.1128/aem.53.9.2212-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apajalahti J. H., Salkinoja-Salonen M. S. Dechlorination and para-hydroxylation of polychlorinated phenols by Rhodococcus chlorophenolicus. J Bacteriol. 1987 Feb;169(2):675–681. doi: 10.1128/jb.169.2.675-681.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. Purification and some properties of a soluble benzene-oxidizing system from a strain of Pseudomonas. Biochem J. 1975 Jan;146(1):173–183. doi: 10.1042/bj1460173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axcell B. C., Geary P. J. The metabolism of benzene by bacteria. Purification and some properties of the enzyme cis-1,2-dihydroxycyclohexa-3,5-diene (nicotinamide adenine dinucleotide) oxidoreductase (cis-benzene glycol dehydrogenase). Biochem J. 1973 Dec;136(4):927–934. doi: 10.1042/bj1360927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brilon C., Beckmann W., Hellwig M., Knackmuss H. J. Enrichment and isolation of naphthalenesulfonic Acid-utilizing pseudomonads. Appl Environ Microbiol. 1981 Jul;42(1):39–43. doi: 10.1128/aem.42.1.39-43.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilon C., Beckmann W., Knackmuss H. J. Catabolism of Naphthalenesulfonic Acids by Pseudomonas sp. A3 and Pseudomonas sp. C22. Appl Environ Microbiol. 1981 Jul;42(1):44–55. doi: 10.1128/aem.42.1.44-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J. P., Kirsch E. J. Metabolism of pentachlorophenol by an axenic bacterial culture. Appl Microbiol. 1972 May;23(5):1033–1035. doi: 10.1128/am.23.5.1033-1035.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxbury J. M., Tiedje J. M., Alexander M., Dawson J. E. 2,4-D metabolism: enzymatic conversion of chloromaleylacetic acid to succinic acid. J Agric Food Chem. 1970 Mar-Apr;18(2):199–201. doi: 10.1021/jf60168a029. [DOI] [PubMed] [Google Scholar]
- Erikson D. Studies on Some Lake-Mud Strains of Micromonospora. J Bacteriol. 1941 Mar;41(3):277–300. doi: 10.1128/jb.41.3.277-300.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathepure B. Z., Tiedje J. M., Boyd S. A. Reductive dechlorination of hexachlorobenzene to tri- and dichlorobenzenes in anaerobic sewage sludge. Appl Environ Microbiol. 1988 Feb;54(2):327–330. doi: 10.1128/aem.54.2.327-330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Harms H., Wittich R. M., Krohn S., Meyer H., Sinnwell V., Wilkes H., Francke W. Metabolism of Dibenzofuran by Pseudomonas sp. Strain HH69 and the Mixed Culture HH27. Appl Environ Microbiol. 1990 Apr;56(4):1148–1156. doi: 10.1128/aem.56.4.1148-1156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Kamenetskii F. Simplification of the empirical relationship between melting temperature of DNA, its GC content and concentration of sodium ions in solution. Biopolymers. 1971;10(12):2623–2624. doi: 10.1002/bip.360101223. [DOI] [PubMed] [Google Scholar]
- Geary P. J., Saboowalla F., Patil D., Cammack R. An investigation of the iron-sulphur proteins of benzene dioxygenase from Pseudomonas putida by electron-spin-resonance spectroscopy. Biochem J. 1984 Feb 1;217(3):667–673. doi: 10.1042/bj2170667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Microbial degradation of halogenated compounds. Science. 1985 Apr 12;228(4696):135–142. doi: 10.1126/science.228.4696.135. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Cardini G. E., Maseles F. C., Kallio R. E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968 Jul;7(7):2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Jerina D. M., Yogi H., Yeh H. J. Oxidation of the carcinogens benzo [a] pyrene and benzo [a] anthracene to dihydrodiols by a bacterium. Science. 1975 Jul 25;189(4199):295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- Haider K., Jagnow G., Kohnen R., Lim S. U. Abbau chlorierter Benzole, Phenole und Cyclohexan-Derivate durch Benzol und Phenol verwertende Bodenbakterien unter aeroben Bedingungen. Arch Microbiol. 1974 Mar 7;96(3):183–200. doi: 10.1007/BF00590175. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of ferredoxinNAP, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):465–468. doi: 10.1128/jb.172.1.465-468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Nishino S. F., Spain J. C. Degradation of 1,2-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1988 Feb;54(2):294–301. doi: 10.1128/aem.54.2.294-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. A., Schlemm D. J., Lyons R. P., 3rd, Sferra P. R., Chakrabarty A. M. Degradation of the chlorinated phenoxyacetate herbicides 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid by pure and mixed bacterial cultures. Appl Environ Microbiol. 1990 May;56(5):1357–1362. doi: 10.1128/aem.56.5.1357-1362.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg S. T., Chatterjee D. K., Chakrabarty A. M. Plasmid-assisted molecular breeding: new technique for enhanced biodegradation of persistent toxic chemicals. Science. 1981 Dec 4;214(4525):1133–1135. doi: 10.1126/science.7302584. [DOI] [PubMed] [Google Scholar]
- Kilbane J. J., Chatterjee D. K., Karns J. S., Kellogg S. T., Chakrabarty A. M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid by a pure culture of Pseudomonas cepacia. Appl Environ Microbiol. 1982 Jul;44(1):72–78. doi: 10.1128/aem.44.1.72-78.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klages U., Markus A., Lingens F. Degradation of 4-chlorophenylacetic acid by a Pseudomonas species. J Bacteriol. 1981 Apr;146(1):64–68. doi: 10.1128/jb.146.1.64-68.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröckel L., Focht D. D. Construction of chlorobenzene-utilizing recombinants by progenitive manifestation of a rare event. Appl Environ Microbiol. 1987 Oct;53(10):2470–2475. doi: 10.1128/aem.53.10.2470-2475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuna M., Someda K., Morita K., Yamanouchi Y., Kurimoto T., Kawamura Y., Matsumura H. [Ischemic cerebral symptoms after subarachnoid hemorrhage due to aneurysmal rupture (author's transl)]. No Shinkei Geka. 1978 Jun;6(6):543–548. [PubMed] [Google Scholar]
- Marinucci A. C., Bartha R. Biodegradation of 1,2,3- and 1,2,4-trichlorobenzene in soil and in liquid enrichment culture. Appl Environ Microbiol. 1979 Nov;38(5):811–817. doi: 10.1128/aem.38.5.811-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A., Klages U., Krauss S., Lingens F. Oxidation and dehalogenation of 4-chlorophenylacetate by a two-component enzyme system from Pseudomonas sp. strain CBS3. J Bacteriol. 1984 Nov;160(2):618–621. doi: 10.1128/jb.160.2.618-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A., Krekel D., Lingens F. Purification and some properties of component A of the 4-chlorophenylacetate 3,4-dioxygenase from Pseudomonas species strain CBS. J Biol Chem. 1986 Sep 25;261(27):12883–12888. [PubMed] [Google Scholar]
- Mires M. H., Alexander C. H. The prophylactic treatment tuberculosis. Del Med J. 1972 Jul;44(7):187–190. [PubMed] [Google Scholar]
- Nakazawa T., Yokota T. Benzoate metabolism in Pseudomonas putida(arvilla) mt-2: demonstration of two benzoate pathways. J Bacteriol. 1973 Jul;115(1):262–267. doi: 10.1128/jb.115.1.262-267.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974 Sep;119(3):879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatello J. J., Martinson M. M., Steiert J. G., Carlson R. E., Crawford R. L. Biodegradation and photolysis of pentachlorophenol in artificial freshwater streams. Appl Environ Microbiol. 1983 Nov;46(5):1024–1031. doi: 10.1128/aem.46.5.1024-1031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial metabolism of haloaromatics: isolation and properties of a chlorobenzene-degrading bacterium. Appl Environ Microbiol. 1984 Feb;47(2):395–402. doi: 10.1128/aem.47.2.395-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem J. 1980 Oct 15;192(1):339–347. doi: 10.1042/bj1920339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraa G., Boone M. L., Jetten M. S., van Neerven A. R., Colberg P. J., Zehnder A. J. Degradation of 1,4-dichlorobenzene by Alcaligenes sp. strain A175. Appl Environ Microbiol. 1986 Dec;52(6):1374–1381. doi: 10.1128/aem.52.6.1374-1381.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer D., Markus A., Seez M., Ruf H. H., Lingens F. Purification and some properties of component B of the 4-chlorophenylacetate 3,4-dioxygenase from Pseudomonas species strain CBS 3. J Biol Chem. 1987 Jul 5;262(19):9340–9346. [PubMed] [Google Scholar]
- Spain J. C., Nishino S. F. Degradation of 1,4-dichlorobenzene by a Pseudomonas sp. Appl Environ Microbiol. 1987 May;53(5):1010–1019. doi: 10.1128/aem.53.5.1010-1019.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittich R. M., Rast H. G., Knackmuss H. J. Degradation of naphthalene-2,6- and naphthalene-1,6-disulfonic acid by a Moraxella sp. Appl Environ Microbiol. 1988 Jul;54(7):1842–1847. doi: 10.1128/aem.54.7.1842-1847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bont J. A., Vorage M. J., Hartmans S., van den Tweel W. J. Microbial degradation of 1,3-dichlorobenzene. Appl Environ Microbiol. 1986 Oct;52(4):677–680. doi: 10.1128/aem.52.4.677-680.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. R., van Neerven A. R., de Vries E. J., de Vos W. M., Zehnder A. J. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J Bacteriol. 1991 Jan;173(1):6–15. doi: 10.1128/jb.173.1.6-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


