Abstract
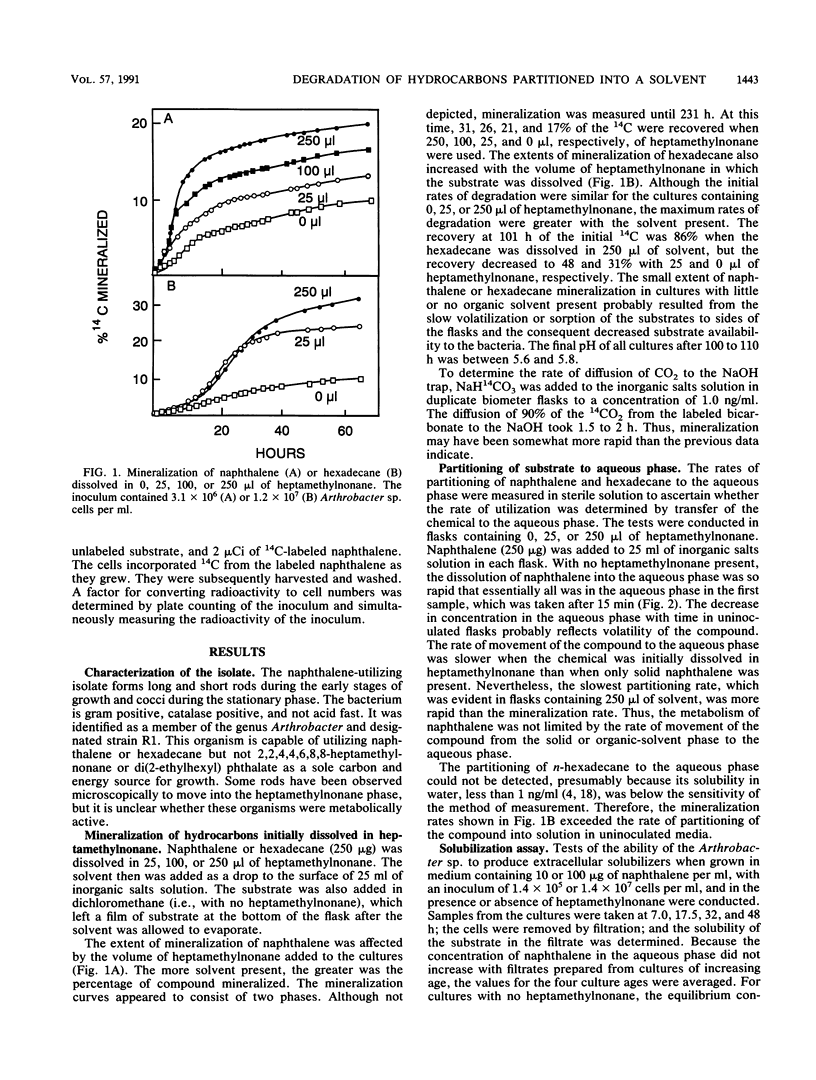
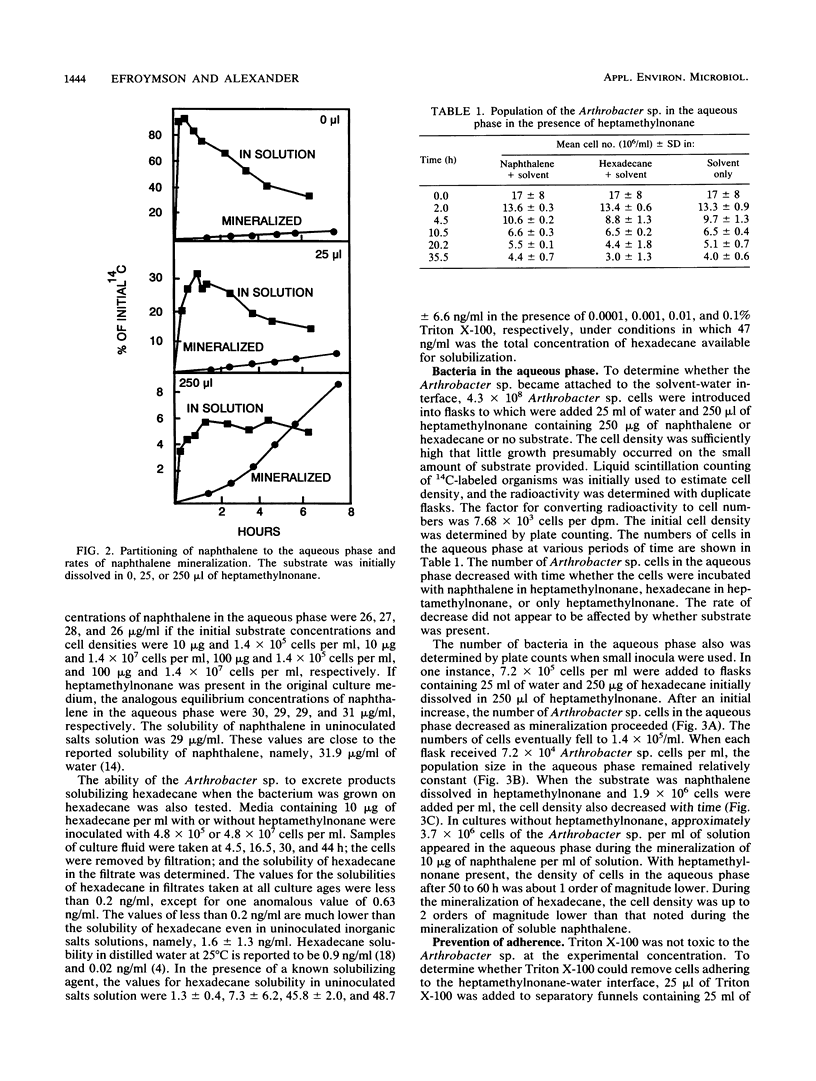
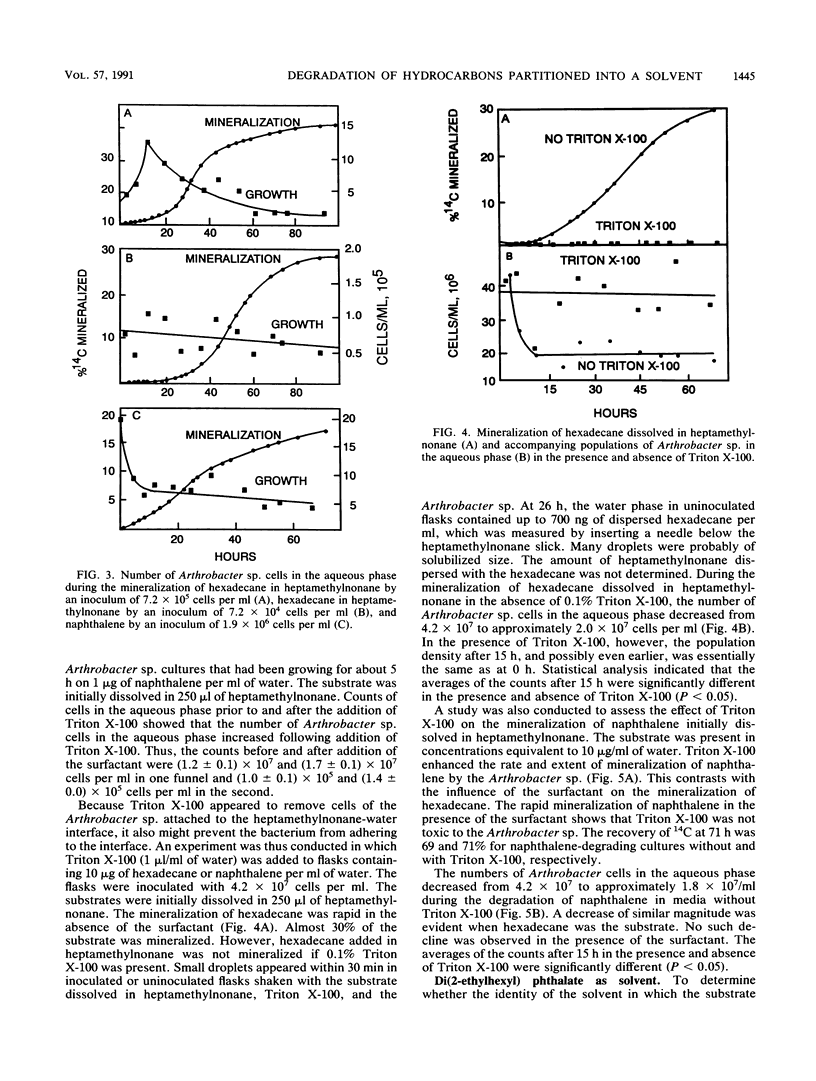
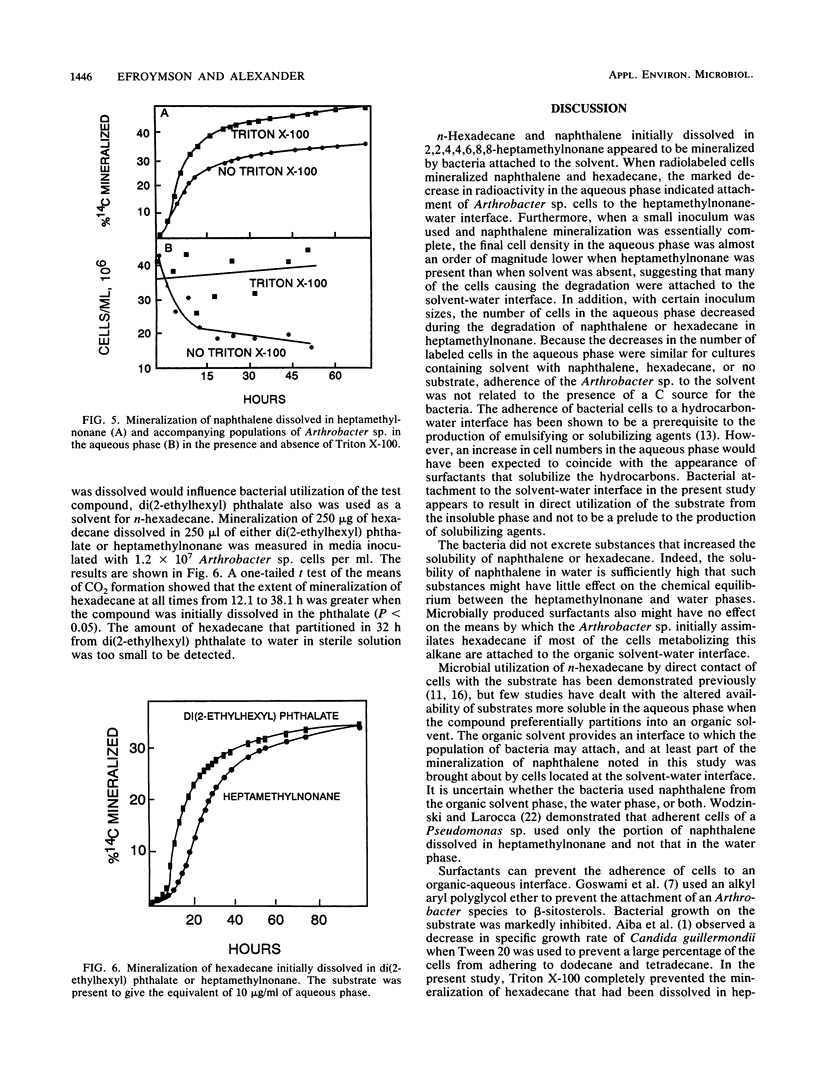
An Arthrobacter strain mineralized naphthalene and n-hexadecane dissolved in 2,2,4,4,6,8,8-heptamethylnonane. The extent of mineralization increased with greater volumes of solvent. Measurements under aseptic conditions of the partitioning of naphthalene into the aqueous phase from the solid phase or from heptamethylnonane showed that the rates were rapid and did not limit mineralization. The rate of mineralization of hexadecane was rapid, although partitioning of the compound into aqueous solution was not detected. The Arthrobacter sp. grown in media with or without heptamethylnonane did not excrete products that increased the aqueous solubility of naphthalene and hexadecane. Measurements of the number of cells in the aqueous phase showed that the Arthrobacter sp. attached to the heptamethylnonane-water interface, but attachment was evident even without a substrate in the heptamethylnonane. Tests with small inocula of the Arthrobacter sp. demonstrated that at least a portion of naphthalene or hexadecane dissolved in heptamethylnonane was degraded by cells attached to the solvent-water interface. The cells did not adhere in the presence of 0.1% Triton X-100. The surfactant prevented mineralization of the hexadecane initially dissolved in heptamethylnonane, but it increased the rate and extent of mineralization of naphthalene initially dissolved in heptamethylnonane. The data show that organic solvents into which hydrophobic compounds partition affect the biodegradation of those compounds and that attachment of microorganisms to the organic solvent-water interface may be important in the transformation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breuil C., Kushner D. J. Effects of lipids, fatty acids, and other detergents on bacterial utilization of hexadecane. Can J Microbiol. 1980 Feb;26(2):223–-31. doi: 10.1139/m80-034. [DOI] [PubMed] [Google Scholar]
- Hoben H. J., Somasegaran P. Comparison of the Pour, Spread, and Drop Plate Methods for Enumeration of Rhizobium spp. in Inoculants Made from Presterilized Peat. Appl Environ Microbiol. 1982 Nov;44(5):1246–1247. doi: 10.1128/aem.44.5.1246-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käppeli O., Walther P., Mueller M., Fiechter A. Structure of the cell surface of the yeast Candida tropicalis and its relation to hydrocarbon transport. Arch Microbiol. 1984 Aug;138(4):279–282. doi: 10.1007/BF00410890. [DOI] [PubMed] [Google Scholar]
- Nakahara T., Erickson L. E., Gutierrez J. R. Characteristics of hydrocarbon uptake in cultures with two liquid phases. Biotechnol Bioeng. 1977 Jan;19(1):9–25. doi: 10.1002/bit.260190103. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Bayer E. A., Delarea J., Rosenberg E. Role of Thin Fimbriae in Adherence and Growth of Acinetobacter calcoaceticus RAG-1 on Hexadecane. Appl Environ Microbiol. 1982 Oct;44(4):929–937. doi: 10.1128/aem.44.4.929-937.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Rosenberg E. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol. 1981 Oct;148(1):51–57. doi: 10.1128/jb.148.1.51-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucki G., Alexander M. Role of dissolution rate and solubility in biodegradation of aromatic compounds. Appl Environ Microbiol. 1987 Feb;53(2):292–297. doi: 10.1128/aem.53.2.292-297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. M., Yordy J. R., Amador J. A., Alexander M. Rates of dissolution and biodegradation of water-insoluble organic compounds. Appl Environ Microbiol. 1986 Aug;52(2):290–296. doi: 10.1128/aem.52.2.290-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzinski R. S., Bertolini D. Physical state in which naphthalene and bibenzyl are utilized by bacteria. Appl Microbiol. 1972 Jun;23(6):1077–1081. doi: 10.1128/am.23.6.1077-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzinski R. S., Coyle J. E. Physical state of phenanthrene for utilization by bacteria. Appl Microbiol. 1974 Jun;27(6):1081–1084. doi: 10.1128/am.27.6.1081-1084.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodzinski R. S., Larocca D. Bacterial growth kinetics on diphenylmethane and naphthalene-heptamethylnonane mixtures. Appl Environ Microbiol. 1977 Mar;33(3):660–665. doi: 10.1128/aem.33.3.660-665.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



