Abstract
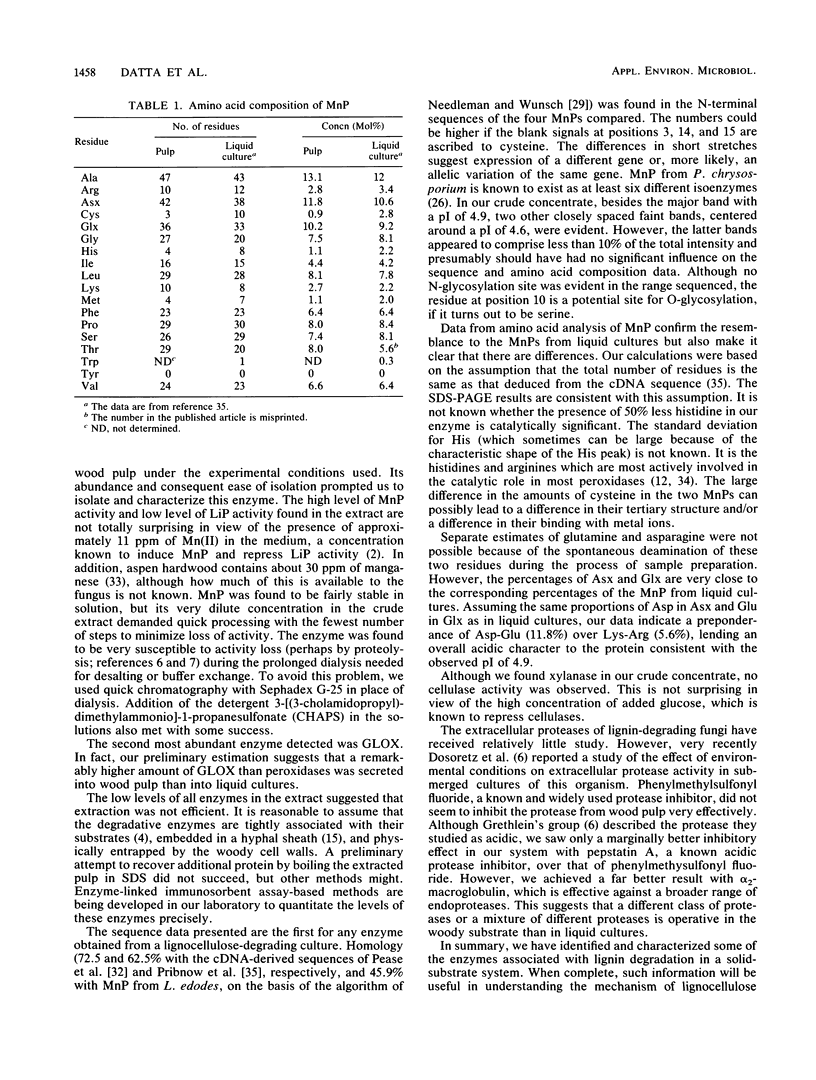
The specific enzymes associated with lignin degradation in solid lignocellulosic substrates have not been identified. Therefore, we examined extracts of cultures of Phanerochaete chrysosporium that were degrading a mechanical pulp of aspen wood. Western blot (immunoblot) analyses of the partially purified protein revealed lignin peroxidase, manganese-dependent peroxidase (MnP), and glyoxal oxidase. The dominant peroxidase, an isoenzyme of MnP (pI 4.9), was isolated, and its N-terminal amino acid sequence and amino acid composition were determined. The results reveal both similarities to and differences from the deduced amino acid sequences from cDNA clones of dominant MnP isoenzymes from liquid cultures. Our results suggest, therefore, that the ligninolytic-enzyme-encoding genes that are expressed during solid substrate degradation differ from those expressed in liquid culture or are allelic variants of their liquid culture counterparts. In addition to lignin peroxidase, MnP, and glyoxal oxidase, xylanase and protease activities were present in the extracts of the degrading pulp.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biely P., Mislovicová D., Toman R. Soluble chromogenic substrates for the assay of endo-1,4-beta-xylanases and endo-1,4-beta-glucanases. Anal Biochem. 1985 Jan;144(1):142–146. doi: 10.1016/0003-2697(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daniel G., Nilsson T., Pettersson B. Intra- and Extracellular Localization of Lignin Peroxidase during the Degradation of Solid Wood and Wood Fragments by Phanerochaete chrysosporium by Using Transmission Electron Microscopy and Immuno-Gold Labeling. Appl Environ Microbiol. 1989 Apr;55(4):871–881. doi: 10.1128/aem.55.4.871-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Chen H. C., Grethlein H. E. Effect of Environmental Conditions on Extracellular Protease Activity in Lignolytic Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Feb;56(2):395–400. doi: 10.1128/aem.56.2.395-400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Dass S. B., Reddy C. A., Grethlein H. E. Protease-mediated degradation of lignin peroxidase in liquid cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Nov;56(11):3429–3434. doi: 10.1128/aem.56.11.3429-3434.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 1. Separation, purification and physico-chemical characterization of five endo-1,4-beta-glucanases. Eur J Biochem. 1975 Feb 3;51(1):193–206. doi: 10.1111/j.1432-1033.1975.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 3. Purification and physico-chemical characterization of an exo-1,4-beta-glucanase. Eur J Biochem. 1975 Feb 3;51(1):213–218. doi: 10.1111/j.1432-1033.1975.tb03921.x. [DOI] [PubMed] [Google Scholar]
- Finzel B. C., Poulos T. L., Kraut J. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-A resolution. J Biol Chem. 1984 Nov 10;259(21):13027–13036. [PubMed] [Google Scholar]
- Forrester I. T., Grabski A. C., Mishra C., Kelley B. D., Strickland W. N., Leatham G. F., Burgess R. R. Characteristics and N-terminal amino acid sequence of a manganese peroxidase purified from Lentinula edodes cultures grown on a commercial wood substrate. Appl Microbiol Biotechnol. 1990 Jun;33(3):359–365. doi: 10.1007/BF00164536. [DOI] [PubMed] [Google Scholar]
- Hammel K. E., Tien M., Kalyanaraman B., Kirk T. K. Mechanism of oxidative C alpha-C beta cleavage of a lignin model dimer by Phanerochaete chrysosporium ligninase. Stoichiometry and involvement of free radicals. J Biol Chem. 1985 Jul 15;260(14):8348–8353. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Kersten P. J. Glyoxal oxidase of Phanerochaete chrysosporium: its characterization and activation by lignin peroxidase. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2936–2940. doi: 10.1073/pnas.87.8.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Leisola M. S., Kozulic B., Meussdoerffer F., Fiechter A. Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem. 1987 Jan 5;262(1):419–424. [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Odier E., Delattre M. Multiple lignin peroxidases of Phanerochaete chrysosporium INA-12. Enzyme Microb Technol. 1990 Jun;12(6):447–452. doi: 10.1016/0141-0229(90)90056-v. [DOI] [PubMed] [Google Scholar]
- Paszczyński A., Huynh V. B., Crawford R. Comparison of ligninase-I and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Feb 1;244(2):750–765. doi: 10.1016/0003-9861(86)90644-2. [DOI] [PubMed] [Google Scholar]
- Pease E. A., Andrawis A., Tien M. Manganese-dependent peroxidase from Phanerochaete chrysosporium. Primary structure deduced from cDNA sequence. J Biol Chem. 1989 Aug 15;264(23):13531–13535. [PubMed] [Google Scholar]
- Poulos T. L., Kraut J. The stereochemistry of peroxidase catalysis. J Biol Chem. 1980 Sep 10;255(17):8199–8205. [PubMed] [Google Scholar]
- Pribnow D., Mayfield M. B., Nipper V. J., Brown J. A., Gold M. H. Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem. 1989 Mar 25;264(9):5036–5040. [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M. Properties of ligninase from Phanerochaete chrysosporium and their possible applications. Crit Rev Microbiol. 1987;15(2):141–168. doi: 10.3109/10408418709104456. [DOI] [PubMed] [Google Scholar]