Abstract
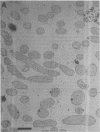
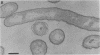
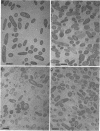
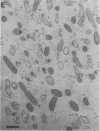
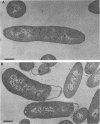
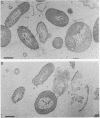
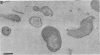
The bacterial isolate MT-41 from 10,476 m, nearly the greatest ocean depth, is obligately barophilic. The purpose of this study was to describe the morphological changes in MT-41 due to nearly isothermal decompression followed by incubation at atmospheric pressure. Two cultures were grown at 103.5 MPa and 2°C and then decompressed to atmospheric pressure (0.101 MPa). One of the cultures was fixed just before decompression. The other culture, kept at 0°C, was sampled immediately and four more times over 168 h. The number of CFU (assayed at 103.5 MPa and 2°C) declined with incubation time at atmospheric pressure. Decompression itself did not lead to immediate morphological changes. The ultrastructure, however, was altered with increasing time at atmospheric pressure. The first aberrations were intracellular vesicles and membrane fragments in the medium. After these changes were plasmolysis, cell lysis, the formation of extracellular vesicles, and the formation of ghost cells. Intact cells in the longest incubation at atmospheric pressure had the normal cytoplasmic granularity suggestive of ribosomes but had few and poorly stained fibrils in the bacterial nucleoids. From the practical standpoint, samples of hadal deep-sea regions need to be fixed either in situ or shortly after arrival at the sea surface even when recovered in insulated sampling gear. This should prevent drastic structural degradation of sampled cells, thus allowing both accurate estimates of deep-sea benthic standing stock and realistic morphological descriptions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braganza L. F., Worcester D. L. Hydrostatic pressure induces hydrocarbon chain interdigitation in single-component phospholipid bilayers. Biochemistry. 1986 May 6;25(9):2591–2596. doi: 10.1021/bi00357a047. [DOI] [PubMed] [Google Scholar]
- DEPETRIS S. ULTRASTRUCTURE OF THE CELL WALL OF ESCHERICHIA COLI. J Ultrastruct Res. 1965 Apr;12:247–262. doi: 10.1016/s0022-5320(65)80098-3. [DOI] [PubMed] [Google Scholar]
- Deming J. W., Hada H., Colwell R. R., Luehrsen K. R., Fox G. E. The ribonucleotide sequence of 5s rRNA from two strains of deep-sea barophilic bacteria. J Gen Microbiol. 1984 Aug;130(8):1911–1920. doi: 10.1099/00221287-130-8-1911. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Hemmingsen B. B., Hemmingsen E. A. Tolerance of bacteria to extreme gas supersaturations. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1379–1384. doi: 10.1016/0006-291x(78)91156-7. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Graves P. N. Stability of free ribosomes, derived ribosomes and polysomes of the sea urchin. Biochim Biophys Acta. 1971 Aug 12;246(1):100–110. doi: 10.1016/0005-2787(71)90075-x. [DOI] [PubMed] [Google Scholar]
- Jannasch H. W., Taylor C. D. Deep-sea microbiology. Annu Rev Microbiol. 1984;38:487–514. doi: 10.1146/annurev.mi.38.100184.002415. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDAU J. V., THIBODEAU L. The micromorphology of Amoeba proteus during pressure-induced changes in the sol-gel cycle. Exp Cell Res. 1962 Sep;27:591–594. doi: 10.1016/0014-4827(62)90027-7. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Chan B., Rose H. M. Electron microscopy of magnesium-depleted bacteria. J Bacteriol. 1966 Feb;91(2):891–895. doi: 10.1128/jb.91.2.891-895.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S. Death of a hadal deep-sea bacterium after decompression. Science. 1983 Apr 29;220(4596):497–498. doi: 10.1126/science.220.4596.497. [DOI] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S. Thermal Inactivation of a Deep-Sea Barophilic Bacterium, Isolate CNPT-3. Appl Environ Microbiol. 1982 Jun;43(6):1481–1489. doi: 10.1128/aem.43.6.1481-1489.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S., VAN Boxtel R. Isolation of a deep-sea barophilic bacterium and some of its growth characteristics. Science. 1979 Aug 24;205(4408):808–810. doi: 10.1126/science.205.4408.808. [DOI] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S., Van Boxtel R. Dependence of reproduction rate on pressure as a hallmark of deep-sea bacteria. Appl Environ Microbiol. 1982 Dec;44(6):1356–1361. doi: 10.1128/aem.44.6.1356-1361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A., Dietz A. S., Van Boxtel R. Obligately barophilic bacterium from the Mariana trench. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5212–5215. doi: 10.1073/pnas.78.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayanos A. A. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9542–9546. doi: 10.1073/pnas.83.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]