Abstract
3-Chlorobenzoate (3Cba)-degrading bacteria were isolated from the waters and sediments of flowthrough mesocosms dosed with various concentrations of 3Cba and inoculated with a 3Cba-degrading Alcaligenes sp., strain BR60. Bacteria capable of 3Cba degradation which were distinct from BR60 were isolated. They carried pBRC60, a plasmid introduced with Alcaligenes sp. strain BR60 that carries a transposable element (Tn5271) encoding 3Cba degradation. The isolates expressed these genes in different ways. The majority of pBRC60 recipients were motile, yellow-pigmented, gram-negative rods related to the group III pseudomonads and to BR60 by substrate utilization pattern. They were capable of complete 3Cba degradation at both millimolar and micromolar concentrations. Two isolates, Pseudomonas fluorescens PR24B(pBRC60) and Pseudomonas sp. strain PR120(pBRC60), are more distantly related to BR60 and both produced chlorocatechol when exposed to 3Cba at millimolar concentrations in the presence of yeast extract. These species showed poor growth in liquid 3Cba minimal medium but could degrade 3Cba in continuous cultures dosed with micromolar levels of the chemical. Laboratory matings confirm that pBRC60 can transfer from BR60 to species in both the beta and gamma subgroups of the proteobacteria and that 3Cba gene expression is variable between species. Selection pressures acting on pBRC60 recipients are discussed.
Full text
PDF

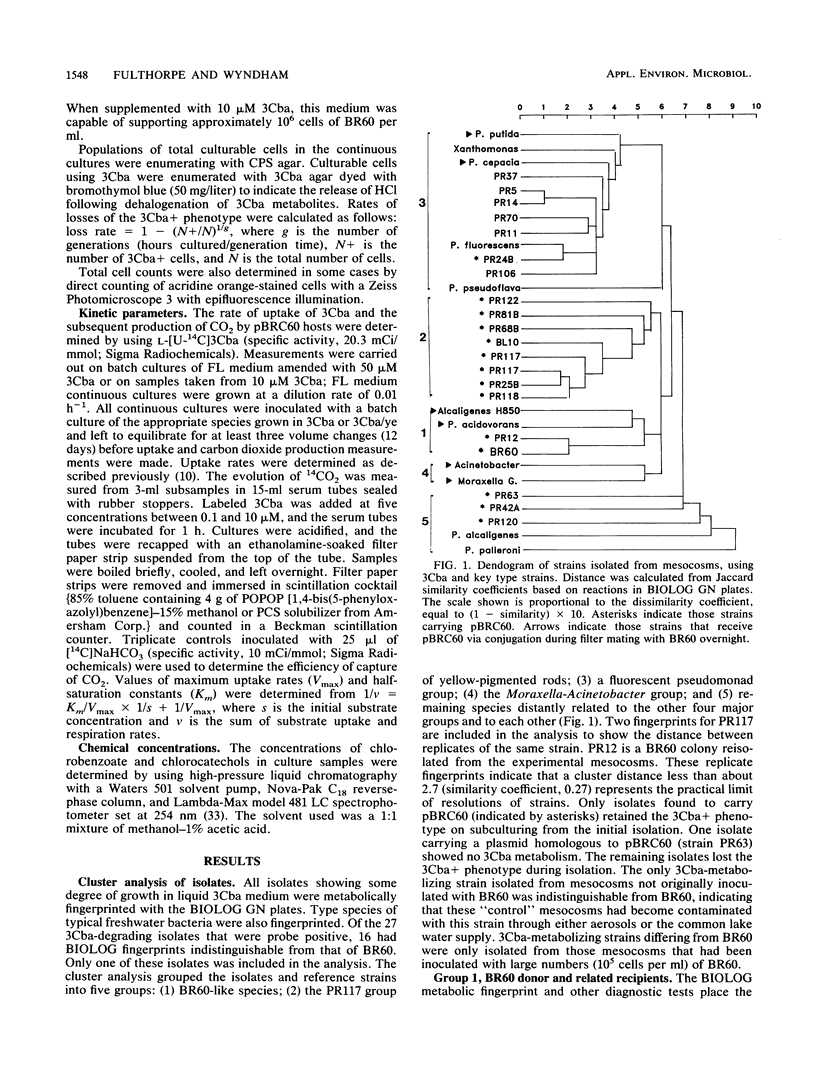
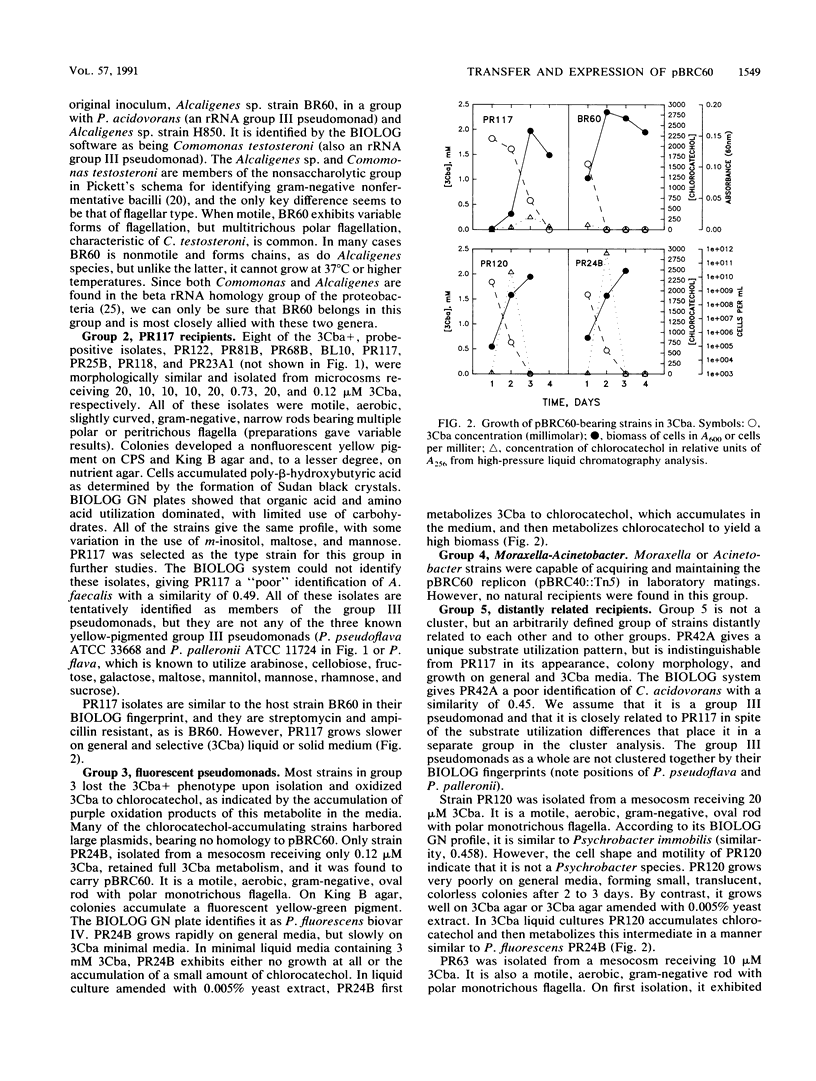
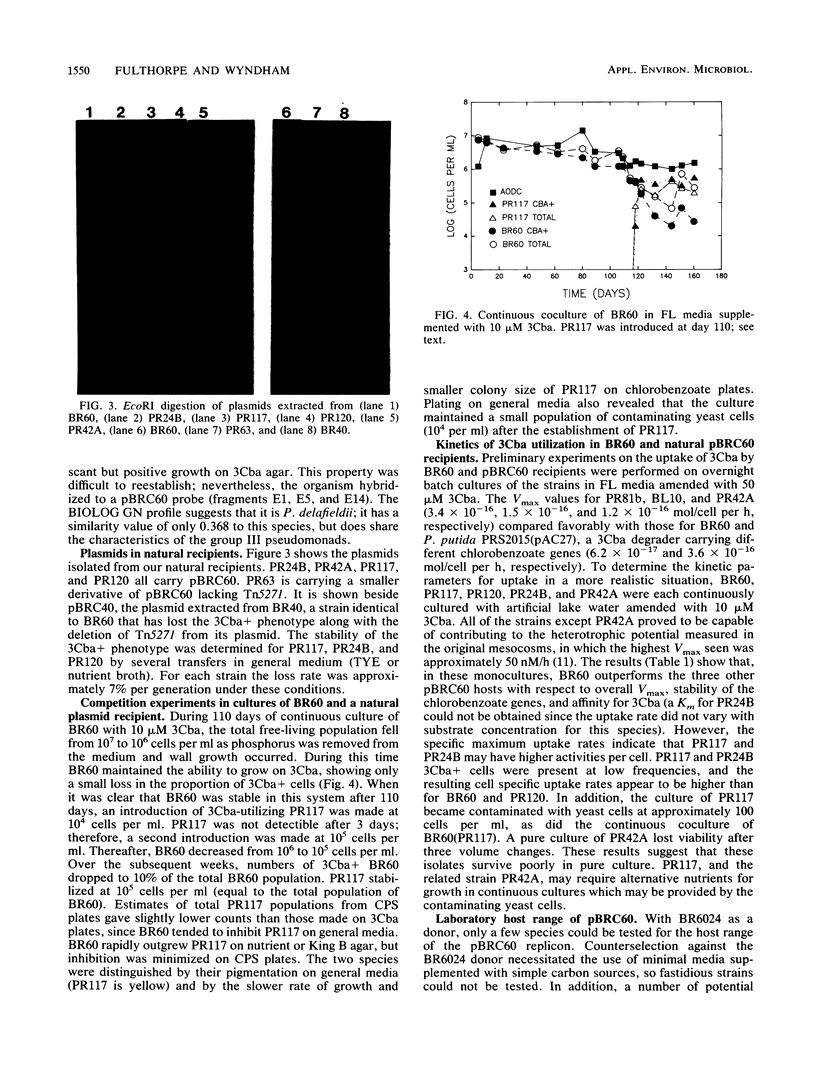


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bale M. J., Fry J. C., Day M. J. Plasmid transfer between strains of Pseudomonas aeruginosa on membrane filters attached to river stones. J Gen Microbiol. 1987 Nov;133(11):3099–3107. doi: 10.1099/00221287-133-11-3099. [DOI] [PubMed] [Google Scholar]
- Caldwell B. A., Ye C., Griffiths R. P., Moyer C. L., Morita R. Y. Plasmid expression and maintenance during long-term starvation-survival of bacteria in well water. Appl Environ Microbiol. 1989 Aug;55(8):1860–1864. doi: 10.1128/aem.55.8.1860-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart G., Hegeman G. Improved method of selection for mutants of Pseudomonas putida. Appl Microbiol. 1975 Dec;30(6):1046–1047. doi: 10.1128/am.30.6.1046-1047.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulcott C. A., Dunn A., Robertson H. A., Cooper N. S., Brown M. E., Rhodes P. M. Investigation of the effect of growth environment on the stability of low-copy-number plasmids in Escherichia coli. J Gen Microbiol. 1987 Jul;133(7):1881–1889. doi: 10.1099/00221287-133-7-1881. [DOI] [PubMed] [Google Scholar]
- Chatfield L. K., Williams P. A. Naturally occurring TOL plasmids in Pseudomonas strains carry either two homologous or two nonhomologous catechol 2,3-oxygenase genes. J Bacteriol. 1986 Nov;168(2):878–885. doi: 10.1128/jb.168.2.878-885.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D. K., Chakrabarty A. M. Genetic homology between independently isolated chlorobenzoate-degradative plasmids. J Bacteriol. 1983 Jan;153(1):532–534. doi: 10.1128/jb.153.1.532-534.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Cruz N. E., Toranzos G. A., Ahearn D. G., Hazen T. C. In situ survival of plasmid-bearing and plasmidless Pseudomonas aeruginosa in pristine tropical waters. Appl Environ Microbiol. 1988 Oct;54(10):2574–2577. doi: 10.1128/aem.54.10.2574-2577.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulthorpe R. R., Wyndham R. C. Survival and activity of a 3-chlorobenzoate-catabolic genotype in a natural system. Appl Environ Microbiol. 1989 Jun;55(6):1584–1590. doi: 10.1128/aem.55.6.1584-1590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealt M. A., Chai M. D., Alpert K. B., Boyer J. C. Transfer of plasmids pBR322 and pBR325 in wastewater from laboratory strains of Escherichia coli to bacteria indigenous to the waste disposal system. Appl Environ Microbiol. 1985 Apr;49(4):836–841. doi: 10.1128/aem.49.4.836-841.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner F. J., Chatterjee P., Barkay T., Bourquin A. W. Capacity of aquatic bacteria to act as recipients of plasmid DNA. Appl Environ Microbiol. 1988 Jan;54(1):115–117. doi: 10.1128/aem.54.1.115-117.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Genes specifying degradation of 3-chlorobenzoic acid in plasmids pAC27 and pJP4. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1638–1642. doi: 10.1073/pnas.82.6.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis Y., Alexander M. Effect of organic amendments on bacterial multiplication in lake water. Antonie Van Leeuwenhoek. 1990 Jan;57(1):9–20. doi: 10.1007/BF00400330. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Klein T. M., Alexander M. Bacterial inhibitors in lake water. Appl Environ Microbiol. 1986 Jul;52(1):114–118. doi: 10.1128/aem.52.1.114-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. W., Edlin G. Expression of tetracycline resistance in pBR322 derivatives reduces the reproductive fitness of plasmid-containing Escherichia coli. Gene. 1985;39(2-3):173–180. doi: 10.1016/0378-1119(85)90311-7. [DOI] [PubMed] [Google Scholar]
- O'Morchoe S. B., Ogunseitan O., Sayler G. S., Miller R. V. Conjugal transfer of R68.45 and FP5 between Pseudomonas aeruginosa strains in a freshwater environment. Appl Environ Microbiol. 1988 Aug;54(8):1923–1929. doi: 10.1128/aem.54.8.1923-1929.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertsova R. N., Kunc F., Golovleva L. A. Degradation of 3-chlorobenzoate in soil by pseudomonads carrying biodegradative plasmids. Folia Microbiol (Praha) 1984;29(3):242–247. doi: 10.1007/BF02877315. [DOI] [PubMed] [Google Scholar]
- Pickett M. J., Pedersen M. M. Nonfermentative bacilli associated with man. II. Detection and identification. Am J Clin Pathol. 1970 Aug;54(2):164–177. doi: 10.1093/ajcp/54.2.164. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuerman P. R., Schmidt J. P., Alexander M. Factors affecting the survival and growth of bacteria introduced into lake water. Arch Microbiol. 1988;150(4):320–325. doi: 10.1007/BF00408301. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotzky G., Babich H. Survival of, and genetic transfer by, genetically engineered bacteria in natural environments. Adv Appl Microbiol. 1986;31:93–138. doi: 10.1016/s0065-2164(08)70440-4. [DOI] [PubMed] [Google Scholar]
- Warnes A., Stephenson J. R. The insertion of large pieces of foreign genetic material reduces the stability of bacterial plasmids. Plasmid. 1986 Sep;16(2):116–123. doi: 10.1016/0147-619x(86)90070-3. [DOI] [PubMed] [Google Scholar]
- Wyndham R. C. Evolved aniline catabolism in Acinetobacter calcoaceticus during continuous culture of river water. Appl Environ Microbiol. 1986 Apr;51(4):781–789. doi: 10.1128/aem.51.4.781-789.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyndham R. C., Singh R. K., Straus N. A. Catabolic instability, plasmid gene deletion and recombination in Alcaligenes sp. BR60. Arch Microbiol. 1988;150(3):237–243. doi: 10.1007/BF00407786. [DOI] [PubMed] [Google Scholar]
- Wyndham R. C., Straus N. A. Chlorobenzoate catabolism and interactions between Alcaligenes and Pseudomonas species from Bloody Run Creek. Arch Microbiol. 1988;150(3):230–236. doi: 10.1007/BF00407785. [DOI] [PubMed] [Google Scholar]
- Zeyer J., Wasserfallen A., Timmis K. N. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol. 1985 Aug;50(2):447–453. doi: 10.1128/aem.50.2.447-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Taxis du Poët P., Arcand Y., Bernier R., Jr, Barbotin J. N., Thomas D. Plasmid stability in immobilized and free recombinant Escherichia coli JM105(pKK223-200): importance of oxygen diffusion, growth rate, and plasmid copy number. Appl Environ Microbiol. 1987 Jul;53(7):1548–1555. doi: 10.1128/aem.53.7.1548-1555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]