Abstract
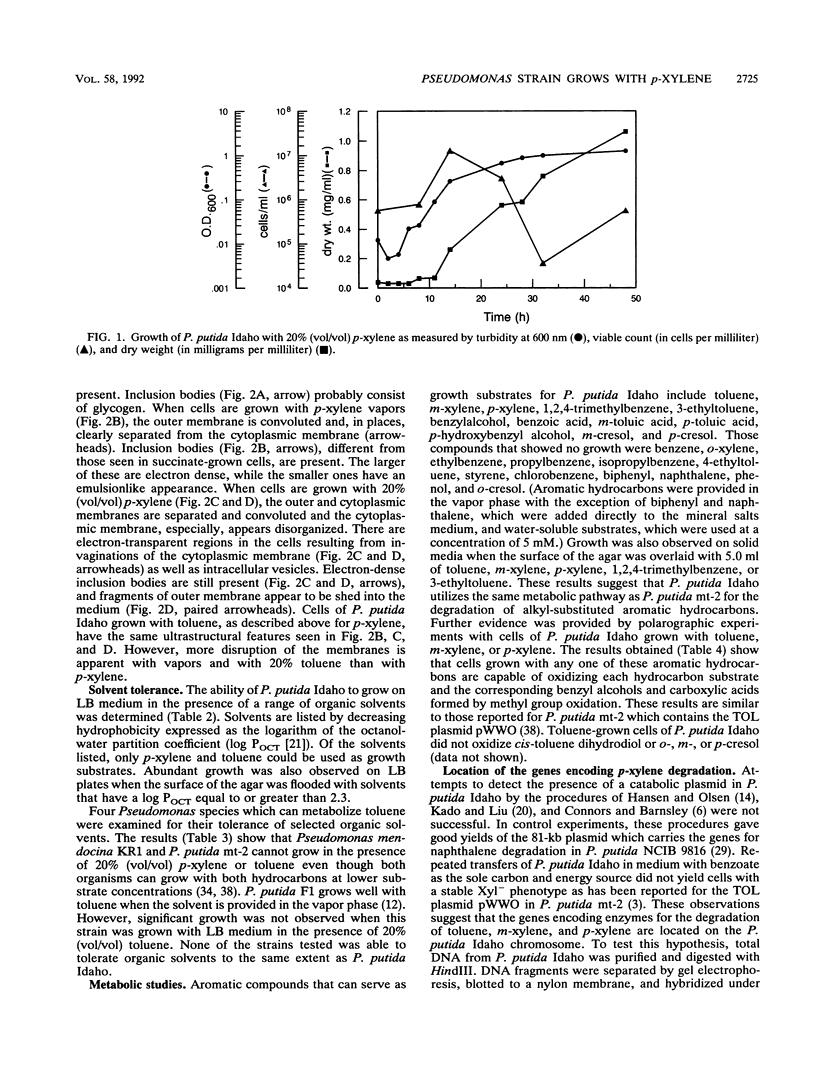
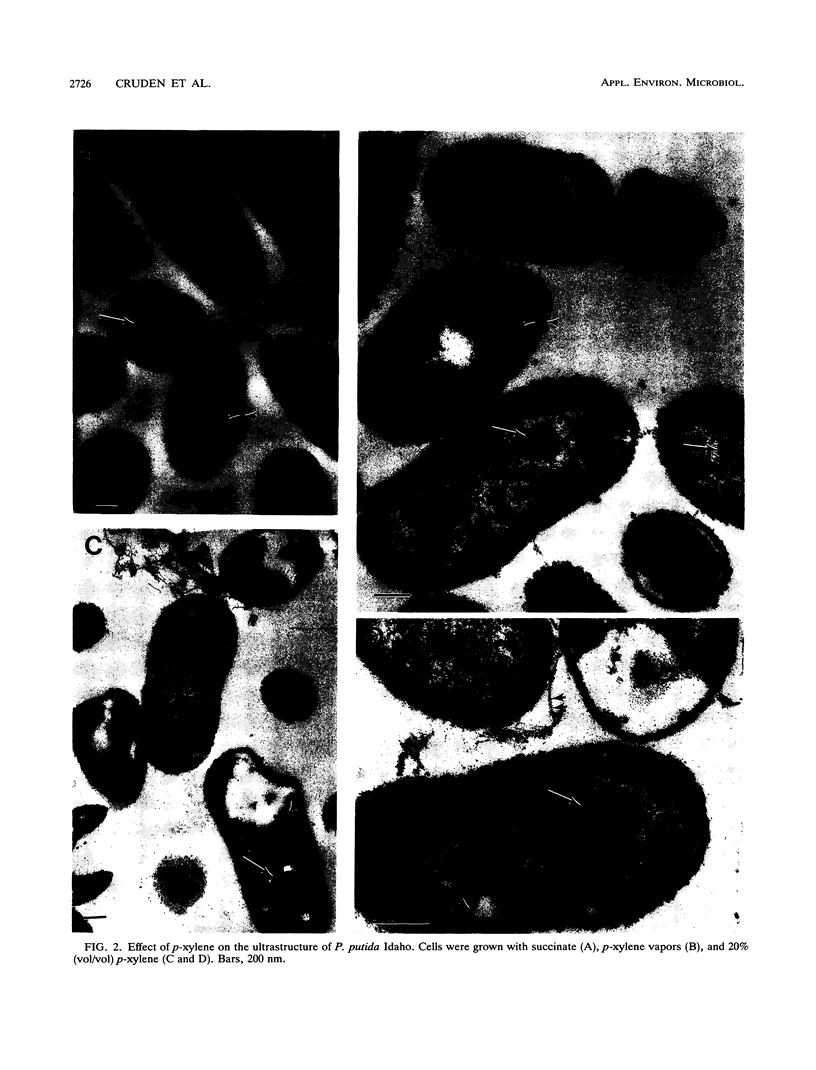
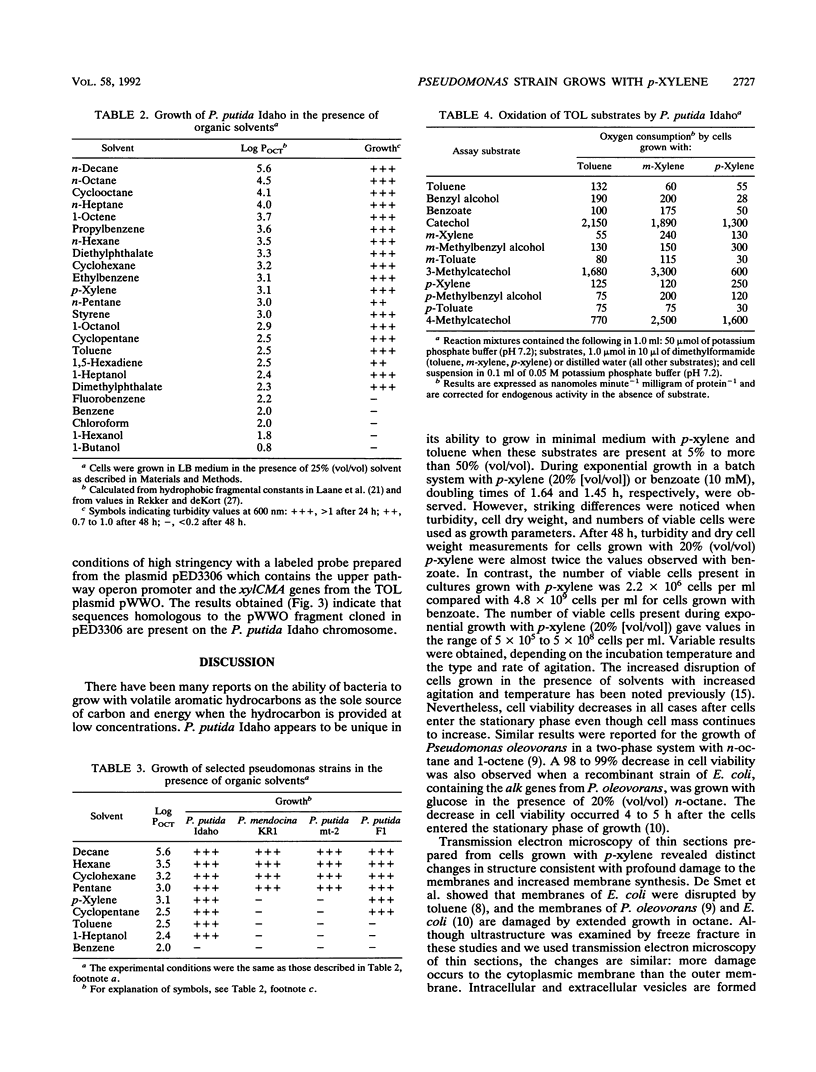
Pseudomonas putida Idaho utilizes toluene, m-xylene, p-xylene, 1,2,4-trimethylbenzene, and 3-ethyltoluene as growth substrates when these hydrocarbons are provided in a two-phase system at 5 to 50% (vol/vol). Growth also occurs on Luria-Bertani medium in the presence of a wide range of organic solvents. The ability of the organism to grow in the presence of organic solvents is correlated with the logarithm of the octanol-water partition coefficient, with dimethyl-phthalate (log P(OCT) = 2.3) being the most polar solvent tolerated. During growth with p-xylene (20% [vol/vol]), there was an initial lag period accompanied by cell death, which was followed by a period of exponential growth. The stationary phase of growth was characterized by a dramatic decrease in cell viability, although cell dry weight and turbidity measurements slowly increased. Electron micrographs revealed that during growth in the presence of p-xylene, the outer cell membrane becomes convoluted and membrane fragments are shed into the culture medium. At the same time, the cytoplasmic membrane invaginates, forming vesicles, and becomes disorganized. Electron-dense intracellular inclusions were observed in cells grown with p-xylene (20% [vol/vol]) and p-xylene vapors, which are not present in cells grown with succinate. Attempts to demonstrate the presence of plasmid DNA in P. putida Idaho were negative. However, polarographic studies indicated that the organism utilizes the same pathway for the degradation of toluene, m-xylene, and p-xylene as that used by P. putida mt-2 which contains the TOL plasmid pWWO.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley S. A., Duggleby C. J., Worsey M. J., Williams P. A., Hardy K. G., Broda P. Two modes of loss of the Tol function from Pseudomonas putida mt-2. Mol Gen Genet. 1977 Jul 20;154(2):203–204. doi: 10.1007/BF00330838. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M. A., Barnsley E. A. Naphthalene plasmids in pseudomonads. J Bacteriol. 1982 Mar;149(3):1096–1101. doi: 10.1128/jb.149.3.1096-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre-Bulle O., Schouten T., Kingma J., Witholt B. Bioconversion of n-octane to octanoic acid by a recombinant Escherichia coli cultured in a two-liquid phase bioreactor. Biotechnology (N Y) 1991 Apr;9(4):367–371. doi: 10.1038/nbt0491-367. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Cardini G. E., Maseles F. C., Kallio R. E. Incorporation of oxygen-18 into benzene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1631–1635. doi: 10.1021/bi00809a024. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Koch J. R., Kallio R. E. Oxidative degradation of aromatic hydrocarbons by microorganisms. I. Enzymatic formation of catechol from benzene. Biochemistry. 1968 Jul;7(7):2653–2662. doi: 10.1021/bi00847a031. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A., Yamamoto M., Horikoshi K. Pseudomonas putida Which Can Grow in the Presence of Toluene. Appl Environ Microbiol. 1991 May;57(5):1560–1562. doi: 10.1128/aem.57.5.1560-1562.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polissi A., Bestetti G., Bertoni G., Galli E., Dehò G. Genetic analysis of chromosomal operons involved in degradation of aromatic hydrocarbons in Pseudomonas putida TMB. J Bacteriol. 1990 Nov;172(11):6355–6362. doi: 10.1128/jb.172.11.6355-6362.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdar C. M., Gibson D. T. Isolation and characterization of altered plasmids in mutant strains of Pseudomonas putida NCIB 9816. Biochem Biophys Res Commun. 1989 Oct 31;164(2):764–771. doi: 10.1016/0006-291x(89)91525-8. [DOI] [PubMed] [Google Scholar]
- Sinclair M. I., Maxwell P. C., Lyon B. R., Holloway B. W. Chromosomal location of TOL plasmid DNA in Pseudomonas putida. J Bacteriol. 1986 Dec;168(3):1302–1308. doi: 10.1128/jb.168.3.1302-1308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Whited G. M., Gibson D. T. Toluene-4-monooxygenase, a three-component enzyme system that catalyzes the oxidation of toluene to p-cresol in Pseudomonas mendocina KR1. J Bacteriol. 1991 May;173(9):3010–3016. doi: 10.1128/jb.173.9.3010-3016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whited G. M., McCombie W. R., Kwart L. D., Gibson D. T. Identification of cis-diols as intermediates in the oxidation of aromatic acids by a strain of Pseudomonas putida that contains a TOL plasmid. J Bacteriol. 1986 Jun;166(3):1028–1039. doi: 10.1128/jb.166.3.1028-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W. Z., Shima Y., Negoro S., Urabe I. Sequence and properties of beta-xylosidase from Bacillus pumilus IPO. Contradiction of the previous nucleotide sequence. Eur J Biochem. 1991 Dec 18;202(3):1197–1203. doi: 10.1111/j.1432-1033.1991.tb16490.x. [DOI] [PubMed] [Google Scholar]
- de Smet M. J., Kingma J., Witholt B. The effect of toluene on the structure and permeability of the outer and cytoplasmic membranes of Escherichia coli. Biochim Biophys Acta. 1978 Jan 4;506(1):64–80. doi: 10.1016/0005-2736(78)90435-2. [DOI] [PubMed] [Google Scholar]