Abstract
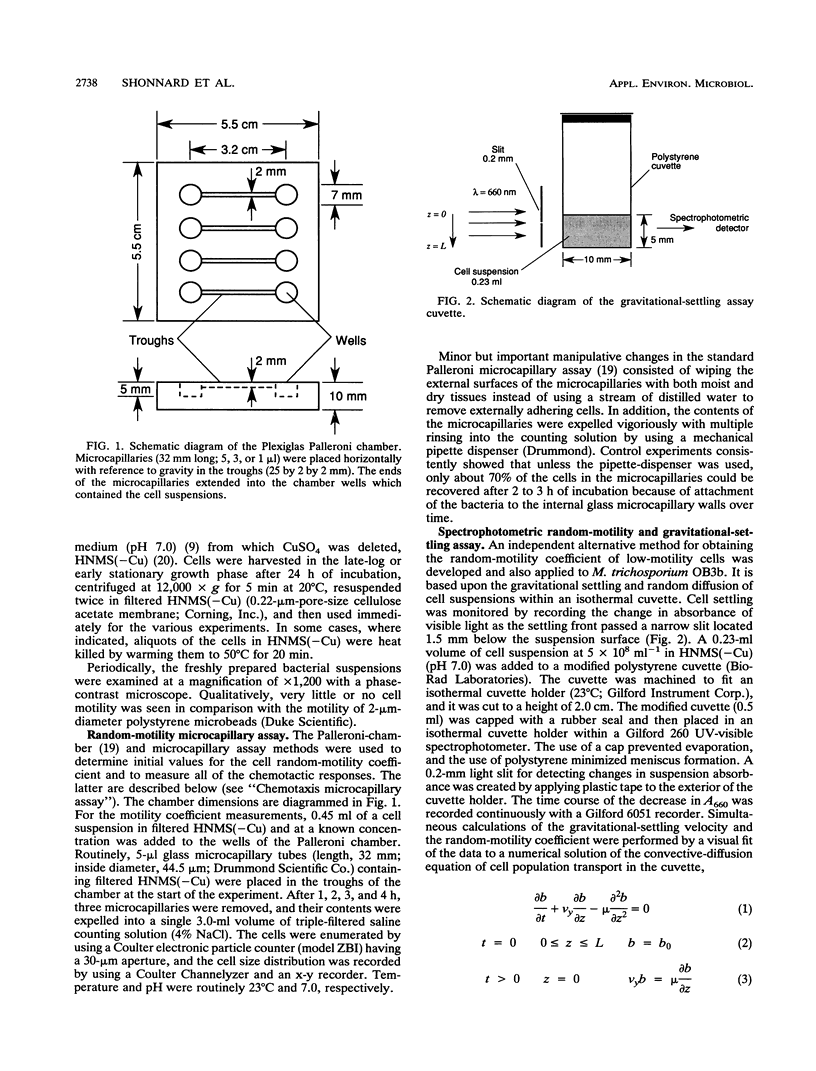
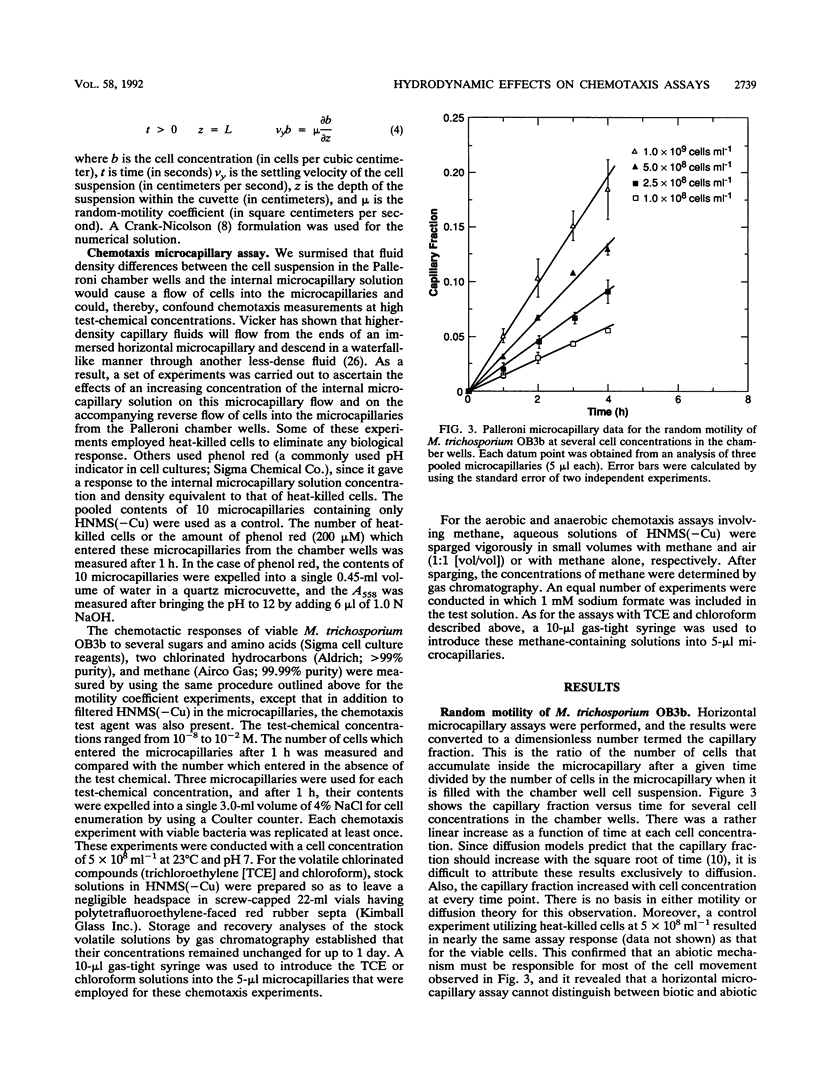
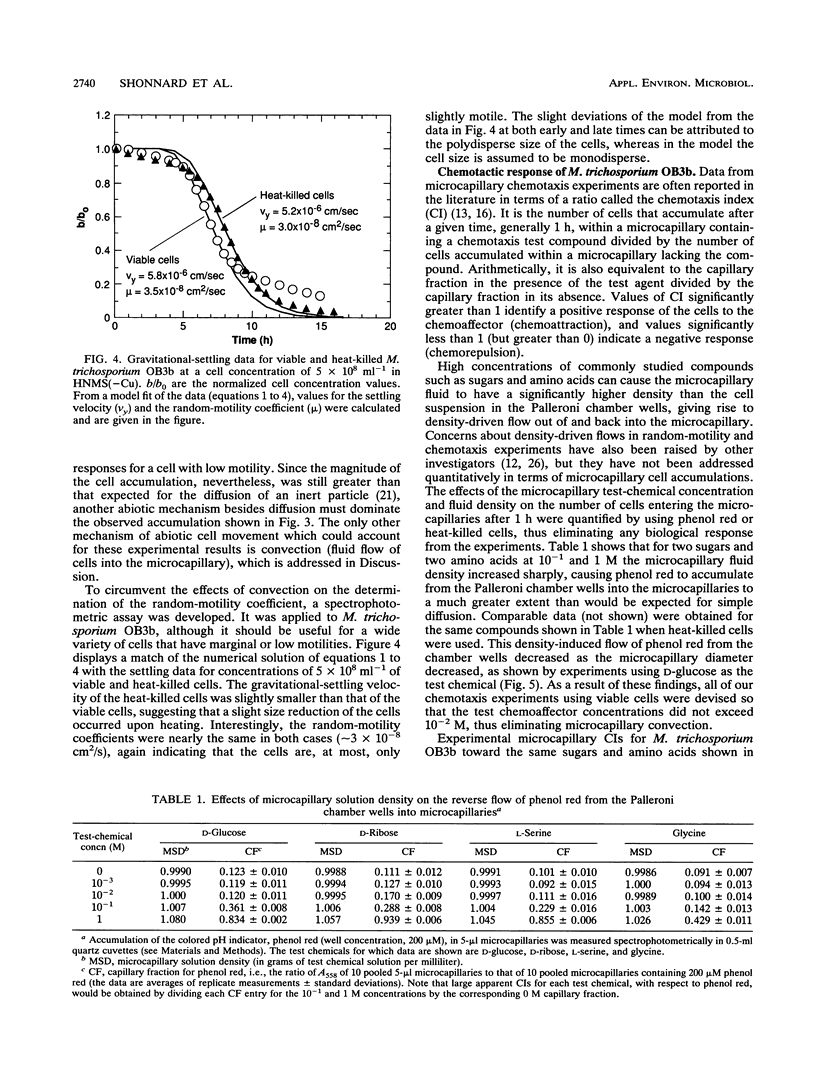
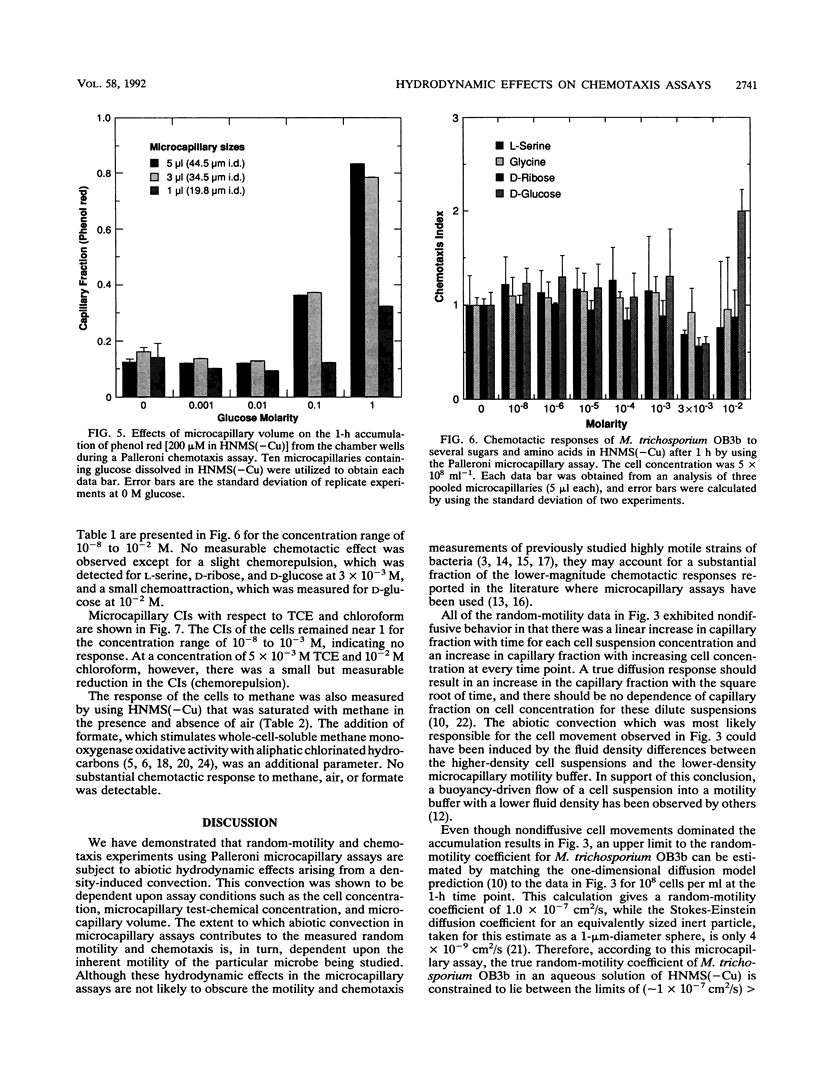
A study of the random motility and chemotaxis of Methylosinus trichosporium OB3b was conducted by using Palleroni-chamber microcapillary assay procedures. Under the growth conditions employed, this methanotroph was observed qualitatively with a microscope to be either slightly motile or essentially nonmotile. However, the cells did not not respond in the microcapillary assays in the manner expected for nonmotile Brownian particles. As a consequence, several hydrodynamic effects on these Palleroni microcapillary assays were uncovered. In the random-motility microcapillary assay, nondiffusive cell accumulations occurred that were strongly dependent upon cell concentration. An apparent minimal random-motility coefficient (mu) for this bacterial cell of 1.0 x 10(-7) cm2/s was estimated from microcapillary assays. A simple alternative spectrophotometric assay, based upon gravitational settling, was developed and shown to be an improvement over the Palleroni microcapillary motility assay for M. trichosporium OB3b in that it yielded a more-accurate threefold-lower random-motility coefficient. In addition, it provided a calculation of the gravitational-settling velocity. In the chemotaxis microcapillary assay, the apparent chemotactic responses were strongest for the highest test-chemical concentrations in the microcapillaries, were correlated with microcapillary fluid density, and were strongly dependent upon the microcapillary volume. A simple method to establish the maximal concentration of a chemical that can be tested and to quantify any contributions of abiotic convection is described. Investigators should be aware of the potential problems due to density-driven convection when using these commonly employed microcapillary assays for studying cells which have low motilities.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Adler J., Dahl M. M. A method for measuring the motility of bacteria and for comparing random and non-random motility. J Gen Microbiol. 1967 Feb;46(2):161–173. doi: 10.1099/00221287-46-2-161. [DOI] [PubMed] [Google Scholar]
- Alvarez-Cohen L., McCarty P. L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991 Jan;57(1):228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist F. W., Lovely P., Koshland D. E., Jr Quantitative analysis of bacterial migration in chemotaxis. Nat New Biol. 1972 Mar 29;236(65):120–123. doi: 10.1038/newbio236120a0. [DOI] [PubMed] [Google Scholar]
- Fuentes F. A., Biamon E. J., Hazen T. C. Bacterial chemotaxis to effluent from a rum distillery in tropical near-shore coastal waters. Appl Environ Microbiol. 1983 Dec;46(6):1438–1441. doi: 10.1128/aem.46.6.1438-1441.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Parales R. E., Dispensa M. Chemotaxis of Pseudomonas putida toward chlorinated benzoates. Appl Environ Microbiol. 1990 May;56(5):1501–1503. doi: 10.1128/aem.56.5.1501-1503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Rivelli M., Ornston L. N. Aromatic acids are chemoattractants for Pseudomonas putida. J Bacteriol. 1984 Nov;160(2):622–628. doi: 10.1128/jb.160.2.622-628.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmcrona-Friberg K., Goodman A., Kjelleberg S. Chemotactic Responses of Marine Vibrio sp. Strain S14 (CCUG 15956) to Low-Molecular-Weight Substances under Starvation and Recovery Conditions. Appl Environ Microbiol. 1990 Dec;56(12):3699–3704. doi: 10.1128/aem.56.12.3699-3704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Vink R. L., Janssen D. B., Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989 Nov;55(11):2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palleroni N. J. Chamber for bacterial chemotaxis experiments. Appl Environ Microbiol. 1976 Nov;32(5):729–730. doi: 10.1128/aem.32.5.729-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel L. A., Chet I., Henis Y. A simple quantitative assay for bacterial motility. J Gen Microbiol. 1977 Feb;98(2):329–337. doi: 10.1099/00221287-98-2-329. [DOI] [PubMed] [Google Scholar]
- Tsien H. C., Brusseau G. A., Hanson R. S., Waclett L. P. Biodegradation of trichloroethylene by Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1989 Dec;55(12):3155–3161. doi: 10.1128/aem.55.12.3155-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicker M. G. Ideal and non-ideal concentration gradient propagation in chemotaxis studies. Exp Cell Res. 1981 Nov;136(1):91–100. doi: 10.1016/0014-4827(81)90040-9. [DOI] [PubMed] [Google Scholar]


