Abstract
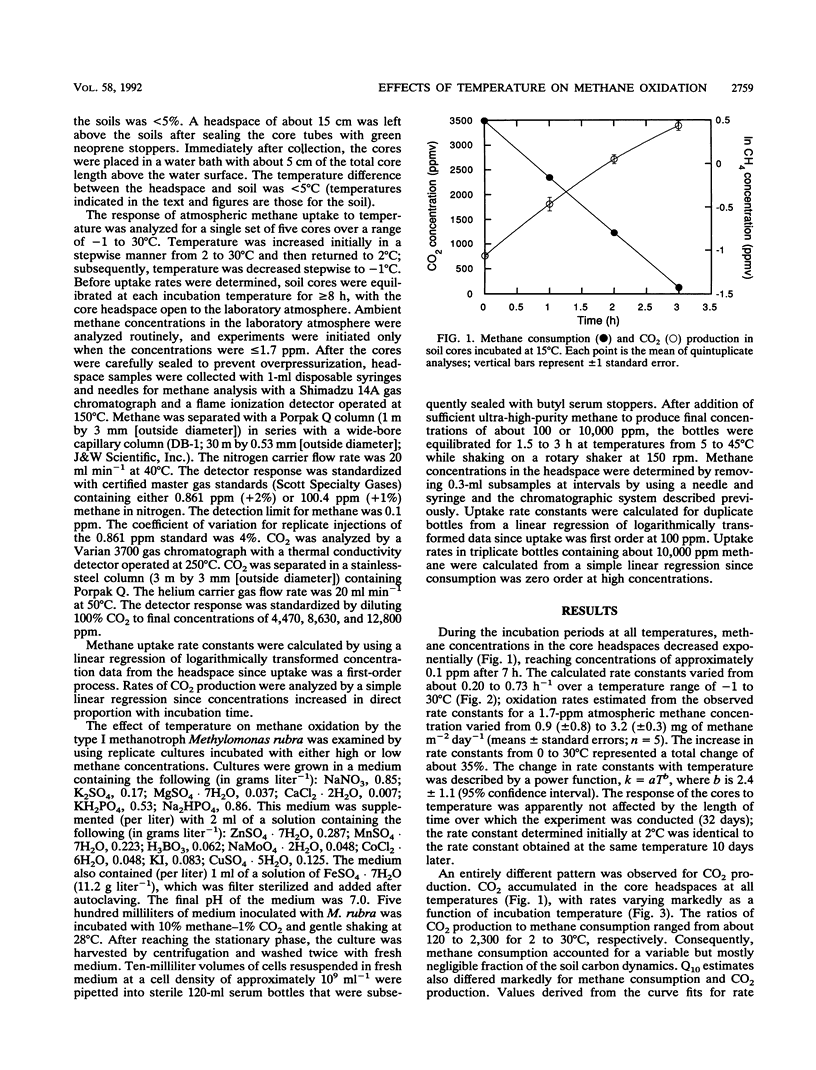
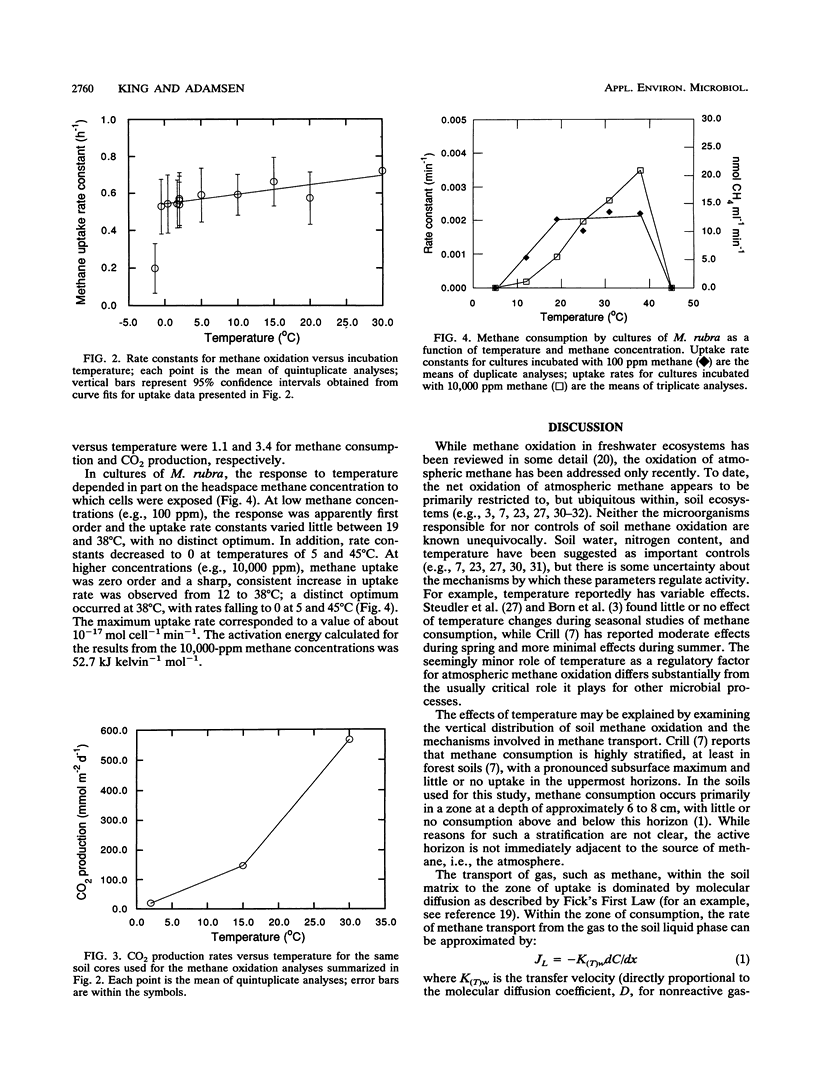
Methane oxidation in soil cores from a mixed hardwood-coniferous forest varied relatively little as a function of incubation temperatures from −1 to 30°C. The increase in oxidation rate was proportional to T2.4 (in kelvins). This relationship was consistent with limitation of methane transport through a soil gas phase to a subsurface zone of consumption by diffusion. The Q10 for CO2 production, 3.4, was substantially higher than that for methane oxidation, 1.1, and indicated that the response of soil respiration to temperature was limited by enzymatic processes and not diffusion of either organic substrates or molecular oxygen. When grown under conditions of phase-transfer limitation, cultures of Methylomonas rubra showed a minimal response to temperature changes between 19 and 38°C, as indicated by methane oxidation rates; in the absence of phase-transfer limitations, M. rubra oxidized methane at rates strongly dependent on temperature.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bédard C., Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989 Mar;53(1):68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R., Meyer O., Seiler W. Role of carboxydobacteria in consumption of atmospheric carbon monoxide by soil. Appl Environ Microbiol. 1981 Aug;42(2):211–215. doi: 10.1128/aem.42.2.211-215.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeu W. Some cultural and physiological aspects of methane-utilizing bacteria. Antonie Van Leeuwenhoek. 1975;41(2):121–134. doi: 10.1007/BF02565044. [DOI] [PubMed] [Google Scholar]
- Hutton W. E., Zobell C. E. THE OCCURRENCE AND CHARACTERISTICS OF METHANE-OXIDIZING BACTERIA IN MARINE SEDIMENTS. J Bacteriol. 1949 Oct;58(4):463–473. doi: 10.1128/jb.58.4.463-473.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. D., Morita R. Y. Methane Oxidation by Nitrosococcus oceanus and Nitrosomonas europaea. Appl Environ Microbiol. 1983 Feb;45(2):401–410. doi: 10.1128/aem.45.2.401-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Tiedje J. M. Kinetics of hydrogen consumption by rumen fluid, anaerobic digestor sludge, and sediment. Appl Environ Microbiol. 1982 Dec;44(6):1374–1384. doi: 10.1128/aem.44.6.1374-1384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen S. C., Reeburgh W. S., Sandbeck K. A. Rapid methane oxidation in a landfill cover soil. Appl Environ Microbiol. 1990 Nov;56(11):3405–3411. doi: 10.1128/aem.56.11.3405-3411.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


