Abstract
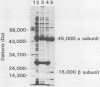
The structural gene coding for the lysine-sensitive aspartokinase II of the methylotrophic thermotolerant Bacillus sp. strain MGA3 was cloned from a genomic library by complementation of an Escherichia coli auxotrophic mutant lacking all three aspartokinase isozymes. The nucleotide sequence of the entire 2.2-kb PstI fragment was determined, and a single open reading frame coding for the aspartokinase II enzyme was found. Aspartokinase II was shown to be an alpha 2 beta 2 tetramer (M(r) 122,000) with the beta subunit (M(r) 18,000) encoded within the alpha subunit (M(r) 45,000) in the samea reading frame. The enzyme was purified, and the N-terminal sequences of the alpha and beta subunits were identical with those predicted from the gene sequences. The predicted amino acid sequence was 76% identical with the sequence of the Bacillus subtilis aspartokinase II. The transcription initiation site was located approximately 350 bp upstream of the translation start site, and putative promoter regions at -10 (TATGCT) and -35 (ATGACA) were identified. A 300-nucleotide intervening sequence between the transcription initiation and translational start sites suggests a possible attenuation mechanism for the regulation of transcription of this enzyme in the presence of lysine.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeles T. S., Smanik P. A., Borders C. L., Jr, Viola R. E. Aspartokinase-homoserine dehydrogenase I from Escherichia coli: pH and chemical modification studies of the kinase activity. Biochemistry. 1989 Oct 31;28(22):8771–8777. doi: 10.1021/bi00448a014. [DOI] [PubMed] [Google Scholar]
- Aota S., Gojobori T., Ishibashi F., Maruyama T., Ikemura T. Codon usage tabulated from the GenBank Genetic Sequence Data. Nucleic Acids Res. 1988;16 (Suppl):r315–r402. doi: 10.1093/nar/16.suppl.r315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band L., Henner D. J. Bacillus subtilis requires a "stringent" Shine-Dalgarno region for gene expression. DNA. 1984;3(1):17–21. doi: 10.1089/dna.1.1984.3.17. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bondaryk R. P., Paulus H. Expression of the gene for Bacillus subtilis aspartokinase II in Escherichia coli. J Biol Chem. 1985 Jan 10;260(1):592–597. [PubMed] [Google Scholar]
- Cassan M., Parsot C., Cohen G. N., Patte J. C. Nucleotide sequence of lysC gene encoding the lysine-sensitive aspartokinase III of Escherichia coli K12. Evolutionary pathway leading to three isofunctional enzymes. J Biol Chem. 1986 Jan 25;261(3):1052–1057. [PubMed] [Google Scholar]
- Cassan M., Ronceray J., Patte J. C. Nucleotide sequence of the promoter region of the E. coli lysC gene. Nucleic Acids Res. 1983 Sep 24;11(18):6157–6166. doi: 10.1093/nar/11.18.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Y., Hu F. M., Paulus H. Nucleotide sequence of the overlapping genes for the subunits of Bacillus subtilis aspartokinase II and their control regions. J Biol Chem. 1987 Jun 25;262(18):8787–8798. [PubMed] [Google Scholar]
- Chen N. Y., Paulus H. Mechanism of expression of the overlapping genes of Bacillus subtilis aspartokinase II. J Biol Chem. 1988 Jul 5;263(19):9526–9532. [PubMed] [Google Scholar]
- Duine J. A., van Dijken J. P. Enzymes of industrial potential from methylotrophs. Biotechnology. 1991;18:233–252. doi: 10.1016/b978-0-7506-9188-8.50017-6. [DOI] [PubMed] [Google Scholar]
- Fazel A., Müller K., Le Bras G., Garel J. R., Véron M., Cohen G. N. A triglobular model for the polypeptide chain of aspartokinase I-homoserine dehydrogenase I of Escherichia coli. Biochemistry. 1983 Jan 4;22(1):158–165. doi: 10.1021/bi00270a023. [DOI] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves L. M., Switzer R. L. Aspartokinase III, a new isozyme in Bacillus subtilis 168. J Bacteriol. 1990 Jan;172(1):218–223. doi: 10.1128/jb.172.1.218-223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton M. L., McCormick N. G., Behforouz N. C., Freese E. Regulation of two aspartokinases in Bacillus subtilis. J Bacteriol. 1971 Dec;108(3):1129–1134. doi: 10.1128/jb.108.3.1129-1134.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Gallagher M. P., Mimmack M. L., Pearce S. R. A family of closely related ATP-binding subunits from prokaryotic and eukaryotic cells. Bioessays. 1988 Apr;8(4):111–116. doi: 10.1002/bies.950080406. [DOI] [PubMed] [Google Scholar]
- Hitchcock M. H., Hodgson B. Lysine- and lysine-plus-threonine-inhibitable aspartokinases in Bacillus brevis. Biochim Biophys Acta. 1976 Sep 14;445(2):350–363. doi: 10.1016/0005-2744(76)90089-9. [DOI] [PubMed] [Google Scholar]
- Katinka M., Cossart P., Sibilli L., Saint-Girons I., Chalvignac M. A., Le Bras G., Cohen G. N., Yaniv M. Nucleotide sequence of the thrA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5730–5733. doi: 10.1073/pnas.77.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M. I., Henner D., Yanofsky C. cis-acting sites in the transcript of the Bacillus subtilis trp operon regulate expression of the operon. J Bacteriol. 1988 Jul;170(7):3080–3088. doi: 10.1128/jb.170.7.3080-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee G., Talkington C., Pero J. Nucleotide sequence of a promoter recognized by Bacillus subtilis RNA polymerase. Mol Gen Genet. 1980;180(1):57–65. doi: 10.1007/BF00267352. [DOI] [PubMed] [Google Scholar]
- Lidstrom M. E., Tsygankov Y. D. Molecular genetics of methylotrophic bacteria. Biotechnology. 1991;18:273–304. doi: 10.1016/b978-0-7506-9188-8.50019-x. [DOI] [PubMed] [Google Scholar]
- Lu Y., Chen N. Y., Paulus H. Identification of aecA mutations in Bacillus subtilis as nucleotide substitutions in the untranslated leader region of the aspartokinase II operon. J Gen Microbiol. 1991 May;137(5):1135–1143. doi: 10.1099/00221287-137-5-1135. [DOI] [PubMed] [Google Scholar]
- Moir D., Paulus H. Properties and subunit structure of aspartokinase II from Bacillus subtilis VB217. J Biol Chem. 1977 Jul 10;252(13):4648–4651. [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Rafalski J. A., Falco S. C. Structure of the yeast HOM3 gene which encodes aspartokinase. J Biol Chem. 1988 Feb 15;263(5):2146–2151. [PubMed] [Google Scholar]
- Rosner A., Paulus H. Regulation of aspartokinase in Bacillus subtilis. The separation and properties of two isofunctional enzymes. J Biol Chem. 1971 May 10;246(9):2965–2971. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel F. J., Bremmon C. E., Flickinger M. C., Guettler M., Hanson R. S. L-lysine production at 50 degrees C by mutants of a newly isolated and characterized methylotrophic Bacillus sp. Appl Environ Microbiol. 1990 Apr;56(4):963–970. doi: 10.1128/aem.56.4.963-970.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Cowe E., Higgins D. G., Shields D. C., Wolfe K. H., Wright F. Codon usage patterns in Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae, Schizosaccharomyces pombe, Drosophila melanogaster and Homo sapiens; a review of the considerable within-species diversity. Nucleic Acids Res. 1988 Sep 12;16(17):8207–8211. doi: 10.1093/nar/16.17.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotsu H., Kuroda M. I., Yanofsky C., Henner D. J. Novel form of transcription attenuation regulates expression the Bacillus subtilis tryptophan operon. J Bacteriol. 1986 May;166(2):461–471. doi: 10.1128/jb.166.2.461-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilli L., Le Bras G., Le Bras G., Cohen G. N. Two regions of the bifunctional protein aspartokinase I- homoserine dehydrogenase I are connected by a short hinge. J Biol Chem. 1981 Oct 25;256(20):10228–10230. [PubMed] [Google Scholar]
- Thèze J., Margarita D., Cohen G. N., Borne F., Patte J. C. Mapping of the structural genes of the three aspartokinases and of the two homoserine dehydrogenases of Escherichia coli K-12. J Bacteriol. 1974 Jan;117(1):133–143. doi: 10.1128/jb.117.1.133-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truffa-Bachi P., Cohen G. N. La beta-aspartokinase sensible à la lysine d'Escherichia coli; purification et propriétés. Biochim Biophys Acta. 1966 Mar 7;113(3):531–541. [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis: evidence for selective induction of prophage in competent cells. J Bacteriol. 1975 Jan;121(1):296–304. doi: 10.1128/jb.121.1.296-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin M. M., Duchange N., Ferrara P., Cohen G. N. Nucleotide sequence of the metL gene of Escherichia coli. Its product, the bifunctional aspartokinase ii-homoserine dehydrogenase II, and the bifunctional product of the thrA gene, aspartokinase I-homoserine dehydrogenase I, derive from a common ancestor. J Biol Chem. 1983 Mar 10;258(5):3028–3031. [PubMed] [Google Scholar]
- Zhang J. J., Hu F. M., Chen N. Y., Paulus H. Comparison of the three aspartokinase isozymes in Bacillus subtilis Marburg and 168. J Bacteriol. 1990 Feb;172(2):701–708. doi: 10.1128/jb.172.2.701-708.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. J., Paulus H. Desensitization of Bacillus subtilis aspartokinase I to allosteric inhibition by meso-diaminopimelate allows aspartokinase I to function in amino acid biosynthesis during exponential growth. J Bacteriol. 1990 Aug;172(8):4690–4693. doi: 10.1128/jb.172.8.4690-4693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]