Abstract
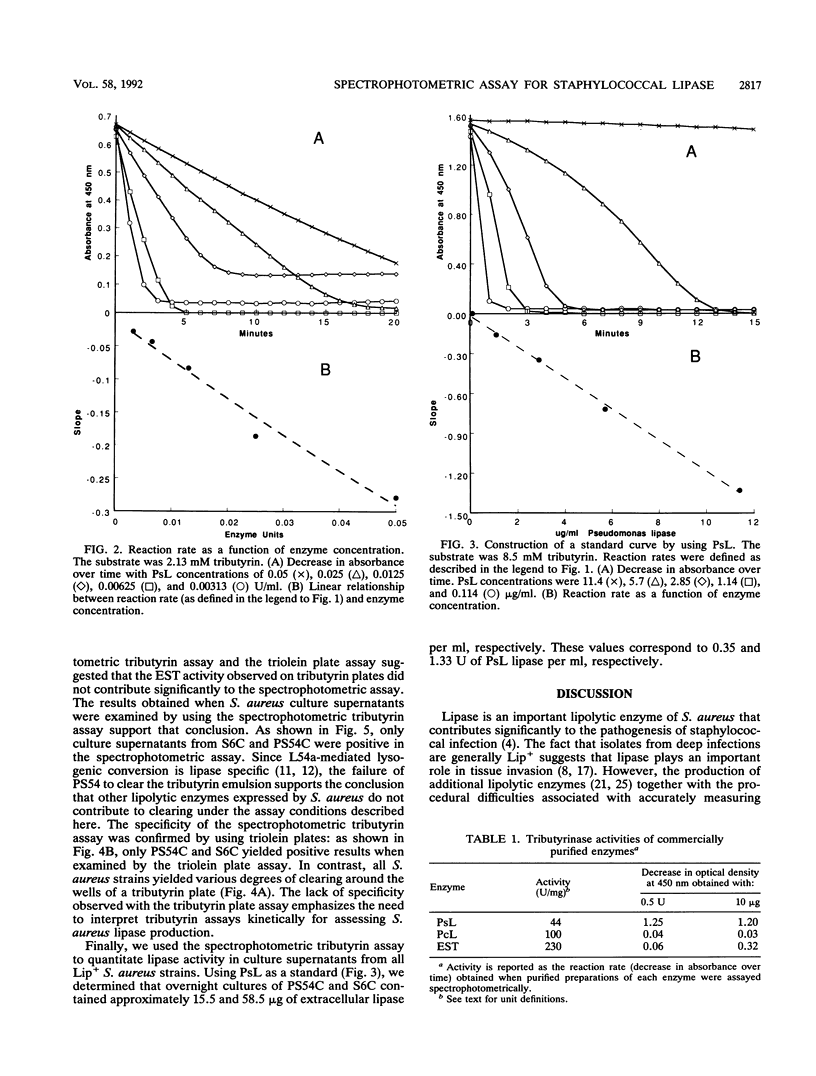
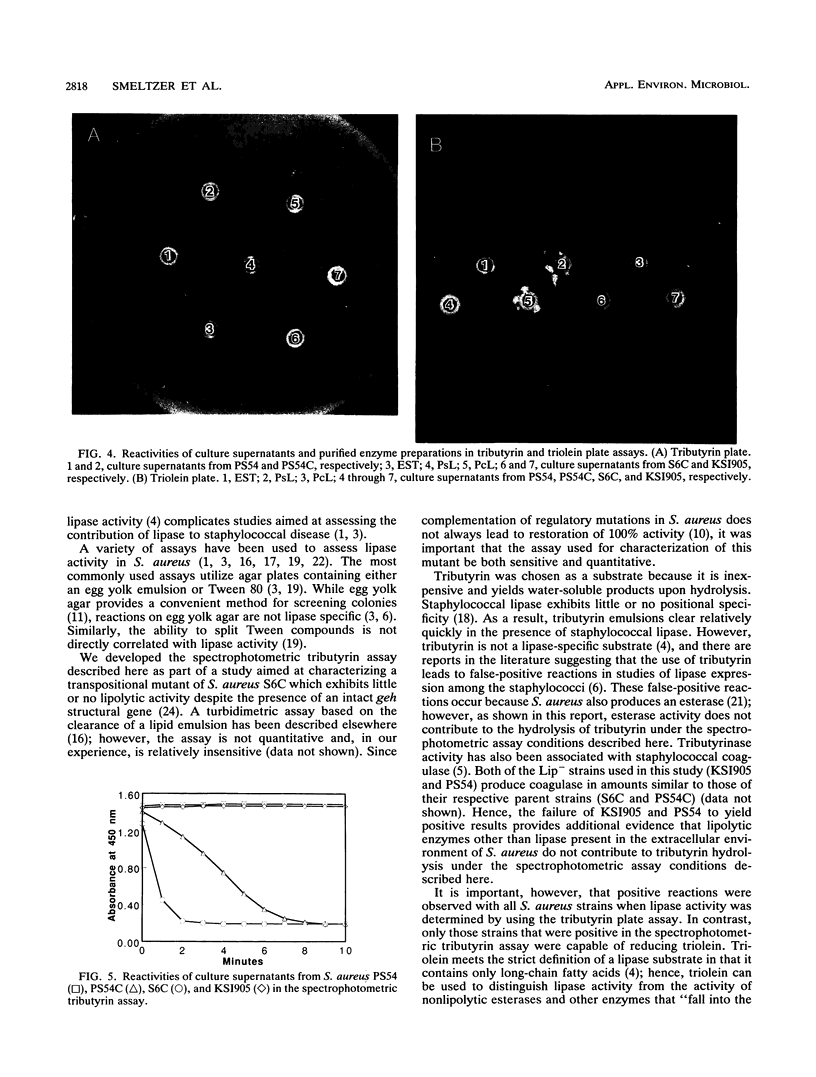
We report the development of a specific spectrophotometric assay for the quantitative determination of lipase activity in Staphylococcus aureus. The assay is based on the rate of clearance of a tributyrin emulsion, and it can detect as little as 1.0 micrograms of purified Pseudomonas lipase per ml. By comparison with the reaction rates obtained with Pseudomonas lipase, we calculated that S. aureus PS54C and S6C produce approximately 15 and 60 micrograms of extracellular lipase per ml, respectively. Neither PS54, which is lysogenized with the converting bacteriophage L54a and is consequently lipase negative (Lip-), nor KS1905, a Lip- transpositional mutant of strain S6C, was positive in our spectrophotometric assay. The specificity of the spectrophotometric tributyrin assay was confirmed with a triolein plate assay; supernatants from S6C and PS54C hydrolyzed triolein, while supernatants from PS54 and KSI905 did not. In contrast to the results of the spectrophotometric tributyrin assay, all enzyme preparations tested (including commercially purified esterase) were positive when examined by a tributyrin plate assay. The lack of specificity in the tributyrin plate assay emphasizes the need to interpret the results of tributyrin lipolysis kinetically for assessing lipase activity in S. aureus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson S., Holme T., Wadström T. Influence of cultivation conditions on the production of extracellular proteins by Staphylococcus aureus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):399–405. doi: 10.1111/j.1699-0463.1971.tb00079.x. [DOI] [PubMed] [Google Scholar]
- DRUMMOND M. C., TAGER M. FIBRINOGEN CLOTTING AND FIBRINO-PEPTIDE FORMATION BY STAPHYLOCOAGULASE AND THE COAGULASE-REACTING FACTOR. J Bacteriol. 1963 Mar;85:628–635. doi: 10.1128/jb.85.3.628-635.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill M. E., Khan S. A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J Biol Chem. 1988 May 5;263(13):6276–6280. [PubMed] [Google Scholar]
- Hedström S. A., Nilsson-Ehle P. Trioleoylglycerol lipolysis by Staphylococcus aureus strains from recurrent furunculosis, pyomyositis, impetigo and osteomyelitis. Acta Pathol Microbiol Immunol Scand B. 1983 Jun;91(3):169–173. doi: 10.1111/j.1699-0463.1983.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Lysogenic conversion of staphylococcal lipase is caused by insertion of the bacteriophage L54a genome into the lipase structural gene. J Bacteriol. 1986 May;166(2):385–391. doi: 10.1128/jb.166.2.385-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Mechanism of bacteriophage conversion of lipase activity in Staphylococcus aureus. J Bacteriol. 1985 Oct;164(1):288–293. doi: 10.1128/jb.164.1.288-293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates A., Sudakevitz D. Production of lipase by Staphylococcus aureus under various growth conditions. J Appl Bacteriol. 1973 Jun;36(2):219–226. doi: 10.1111/j.1365-2672.1973.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Rollof J., Braconier J. H., Söderström C., Nilsson-Ehle P. Interference of Staphylococcus aureus lipase with human granulocyte function. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):505–510. doi: 10.1007/BF01962601. [DOI] [PubMed] [Google Scholar]
- Rollof J., Hedström S. A., Nilsson-Ehle P. A simple turbidimetric method for specific measurement of Staphylococcus aureus lipase activity. Acta Pathol Microbiol Immunol Scand B. 1984 Jun;92(3):155–158. doi: 10.1111/j.1699-0463.1984.tb02811.x. [DOI] [PubMed] [Google Scholar]
- Rollof J., Hedström S. A., Nilsson-Ehle P. Lipolytic activity of Staphylococcus aureus strains from disseminated and localized infections. Acta Pathol Microbiol Immunol Scand B. 1987 Apr;95(2):109–113. doi: 10.1111/j.1699-0463.1987.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Rollof J., Hedström S. A., Nilsson-Ehle P. Positional specificity and substrate preference of purified Staphylococcus aureus lipase. Biochim Biophys Acta. 1987 Sep 25;921(2):370–377. doi: 10.1016/0005-2760(87)90039-7. [DOI] [PubMed] [Google Scholar]
- Rollof J., Hedström S. A., Nilsson-Ehle P. The Tween 80 reaction does not correlate to triglyceride lipase production of Staphylococcus aureus. APMIS. 1988 Aug;96(8):732–734. doi: 10.1111/j.1699-0463.1988.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Rollof J., Normark S. In vivo processing of Staphylococcus aureus lipase. J Bacteriol. 1992 Mar;174(6):1844–1847. doi: 10.1128/jb.174.6.1844-1847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggers B. A., Stewart G. T. Lipolytic esterases in staphylococci. J Bacteriol. 1968 Oct;96(4):1006–1010. doi: 10.1128/jb.96.4.1006-1010.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Clemente C. L., Vadehra D. V. Instrumental assay of microbial lipase at constant pH. Appl Microbiol. 1967 Jan;15(1):110–113. doi: 10.1128/am.15.1.110-113.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer M. S., Gill S. R., Iandolo J. J. Localization of a chromosomal mutation affecting expression of extracellular lipase in Staphylococcus aureus. J Bacteriol. 1992 Jun;174(12):4000–4006. doi: 10.1128/jb.174.12.4000-4006.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. T. The lipases and pigments of staphylococci. Ann N Y Acad Sci. 1965 Jul 23;128(1):132–151. doi: 10.1111/j.1749-6632.1965.tb11635.x. [DOI] [PubMed] [Google Scholar]



