Abstract
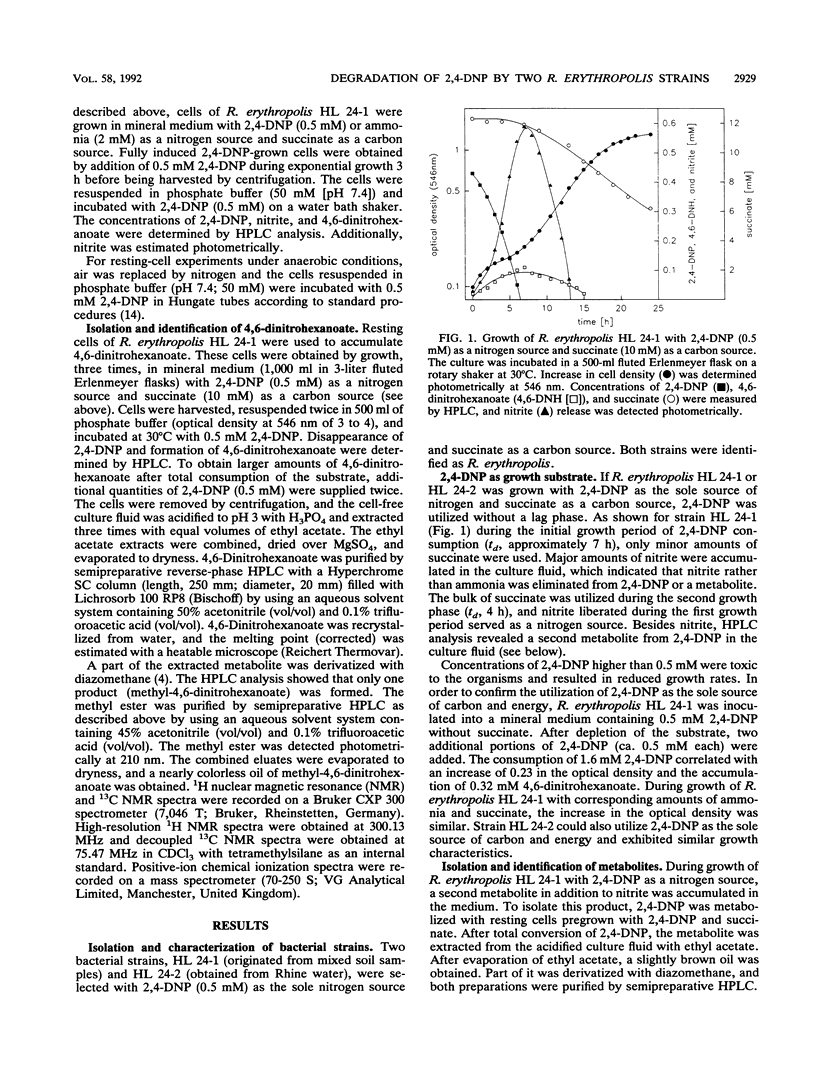
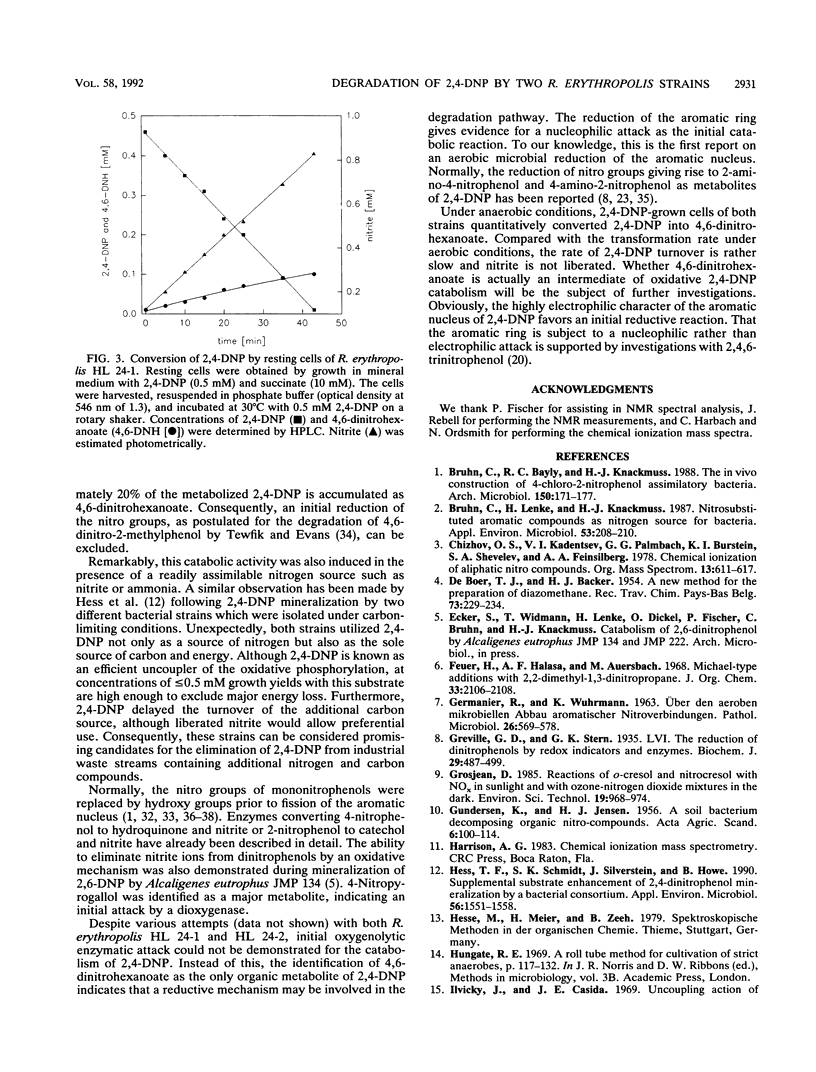
Two Rhodococcus erythropolis strains, HL 24-1 and HL 24-2, were isolated from soil and river water by their abilities to utilize 2,4-dinitrophenol (0.5 mM) as the sole source of nitrogen. Although succinate was supplied as a carbon and energy source during selection, both isolates could utilize 2,4-dinitrophenol also as the sole source of carbon. Both strains metabolized 2,4-dinitrophenol under concomitant liberation of stoichiometric amounts of nitrite and 4,6-dinitrohexanoate as a minor dead-end metabolite.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruhn C., Lenke H., Knackmuss H. J. Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl Environ Microbiol. 1987 Jan;53(1):208–210. doi: 10.1128/aem.53.1.208-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERMANIER R., WUHRMANN K. UBER DEN AEROBEN MIKROBIELLEN ABBAU AROMATISCHER NITROVERBINDUNGEN. Pathol Microbiol (Basel) 1963;26:569–578. [PubMed] [Google Scholar]
- Greville G. D., Stern K. G. The reduction of dinitrophenols by redox indicators and enzymes. Biochem J. 1935 Feb;29(2):487–499. doi: 10.1042/bj0290487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess T. F., Schmidt S. K., Silverstein J., Howe B. Supplemental substrate enhancement of 2,4-dinitrophenol mineralization by a bacterial consortium. Appl Environ Microbiol. 1990 Jun;56(6):1551–1558. doi: 10.1128/aem.56.6.1551-1558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN H. L., GUNDERSEN K. Biological decomposition of aromatic nitro-compounds. Nature. 1955 Feb 19;175(4451):341–341. doi: 10.1038/175341a0. [DOI] [PubMed] [Google Scholar]
- Kaplan A. The determination of urea, ammonia, and urease. Methods Biochem Anal. 1969;17:311–324. doi: 10.1002/9780470110355.ch7. [DOI] [PubMed] [Google Scholar]
- Lenke H., Knackmuss H. J. Initial hydrogenation during catabolism of picric acid by Rhodococcus erythropolis HL 24-2. Appl Environ Microbiol. 1992 Sep;58(9):2933–2937. doi: 10.1128/aem.58.9.2933-2937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADHOSINGH C. The metabolic detoxication of 2,4-dinitrophenol by Fusarium oxysporum. Can J Microbiol. 1961 Aug;7:553–567. doi: 10.1139/m61-065. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Gibson D. T. Pathway for Biodegradation of p-Nitrophenol in a Moraxella sp. Appl Environ Microbiol. 1991 Mar;57(3):812–819. doi: 10.1128/aem.57.3.812-819.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Wyss O., Gibson D. T. Enzymatic oxidation of p-nitrophenol. Biochem Biophys Res Commun. 1979 May 28;88(2):634–641. doi: 10.1016/0006-291x(79)92095-3. [DOI] [PubMed] [Google Scholar]
- WESTERFELD W. W., RICHERT D. A., HIGGINS E. S. The metabolic reduction of organic nitro groups. J Biol Chem. 1957 Jul;227(1):379–391. [PubMed] [Google Scholar]
- Zeyer J., Kocher H. P. Purification and characterization of a bacterial nitrophenol oxygenase which converts ortho-nitrophenol to catechol and nitrite. J Bacteriol. 1988 Apr;170(4):1789–1794. doi: 10.1128/jb.170.4.1789-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Kocher H. P., Timmis K. N. Influence of para-substituents on the oxidative metabolism of o-nitrophenols by Pseudomonas putida B2. Appl Environ Microbiol. 1986 Aug;52(2):334–339. doi: 10.1128/aem.52.2.334-339.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


