Abstract
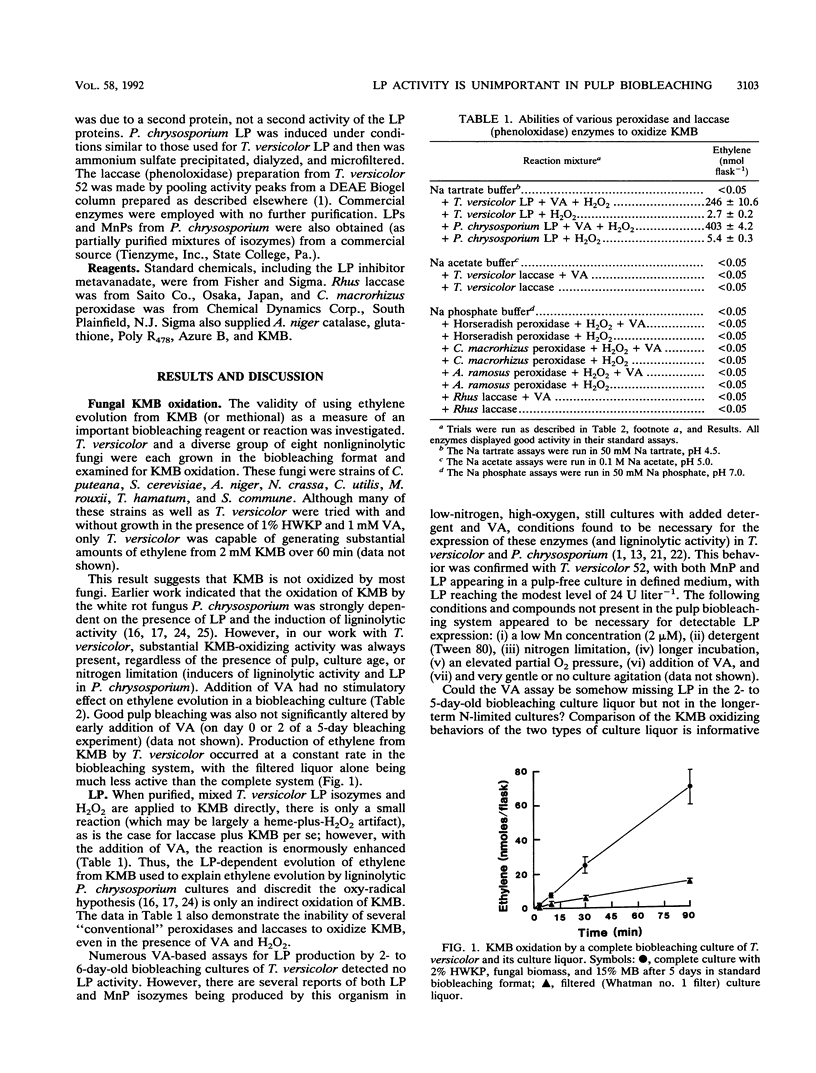
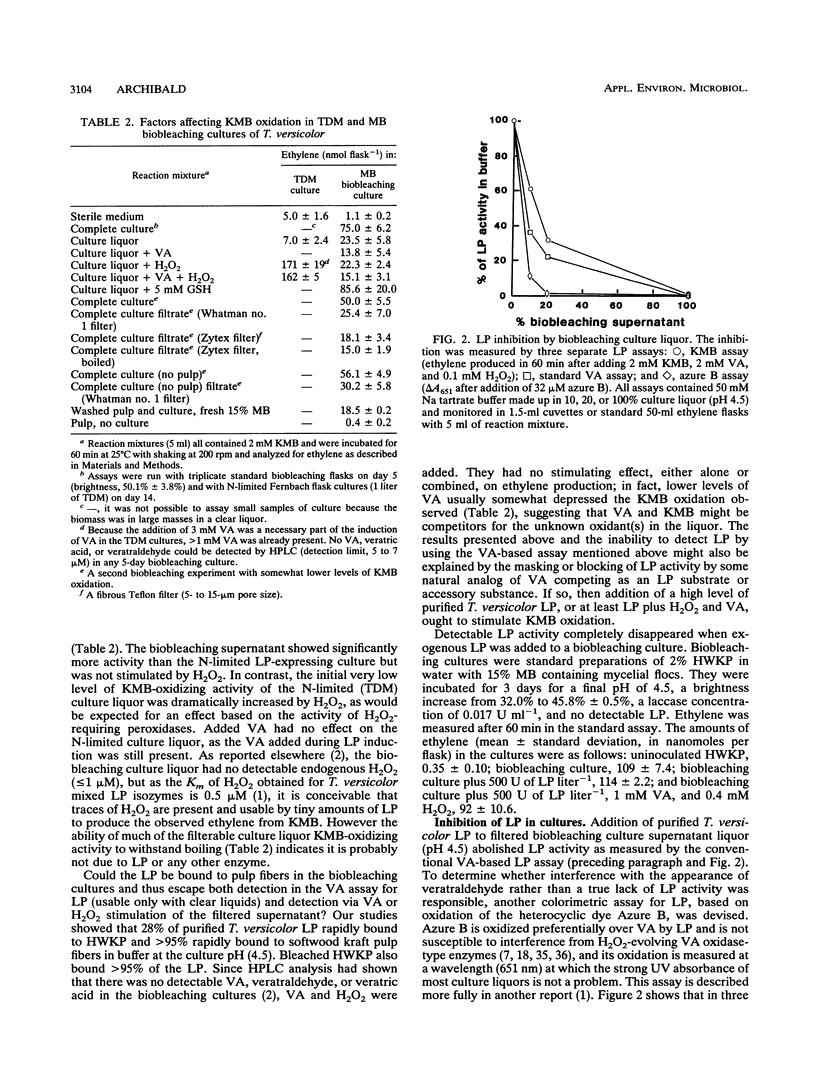
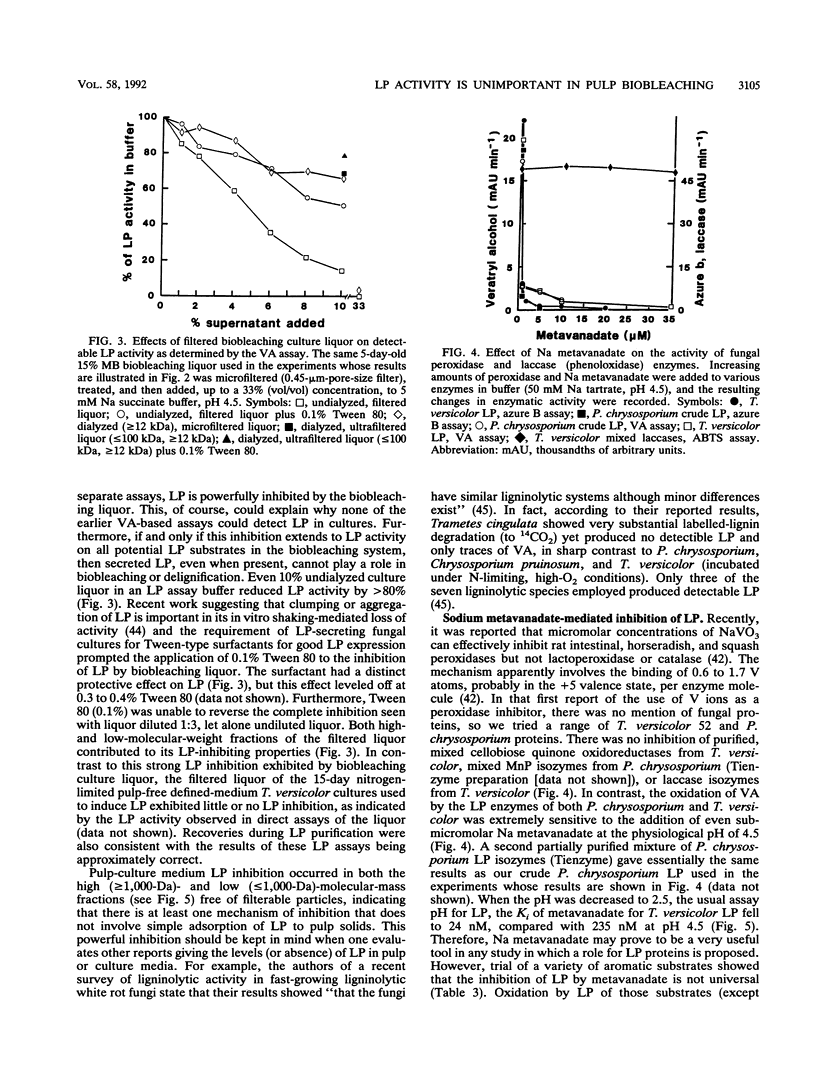
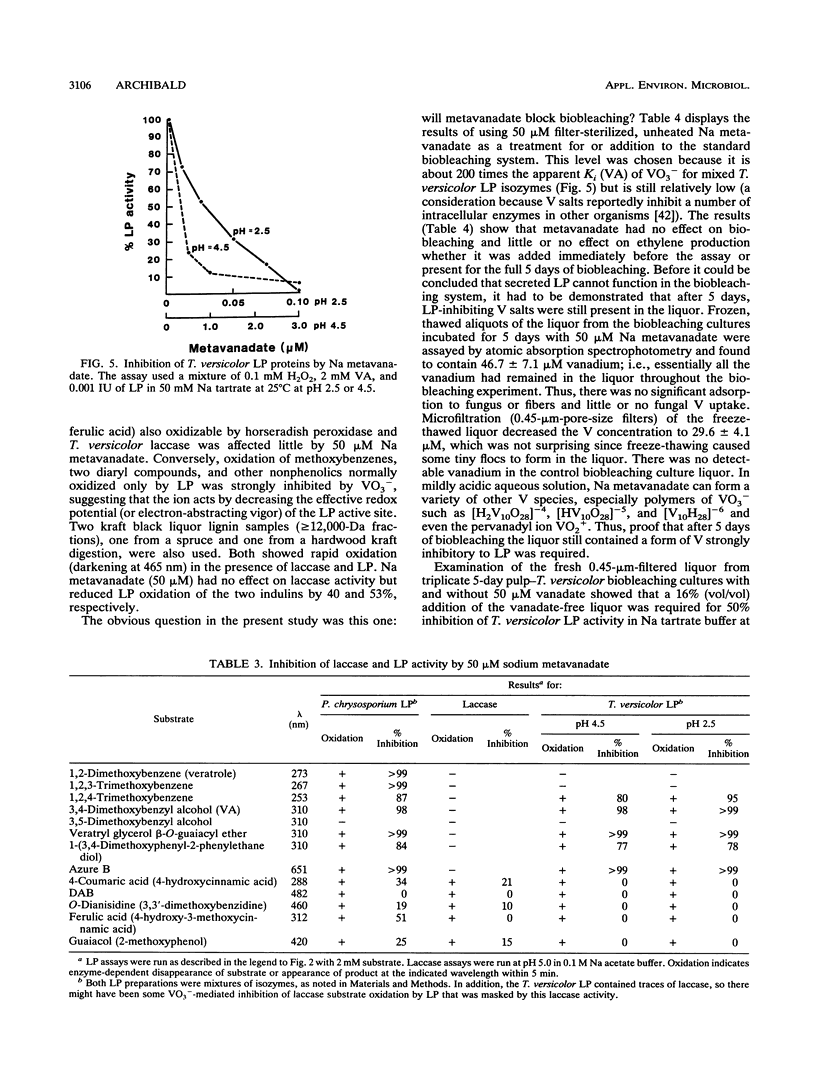
The discovery in 1983 of fungal lignin peroxidases able to catalyze the oxidation of nonphenolic aromatic lignin model compounds and release some CO2 from lignin has been seen as a major advance in understanding how fungi degrade lignin. Recently, the fungus Trametes versicolor was shown to be capable of substantial decolorization and delignification of unbleached industrial kraft pulps over 2 to 5 days. The role, if any, of lignin peroxidase in this biobleaching was therefore examined. Several different assays indicated that T. versicolor can produce and secrete peroxidase proteins, but only under certain culture conditions. However, work employing a new lignin peroxidase inhibitor (metavanadate ions) and a new lignin peroxidase assay using the dye azure B indicated that secreted lignin peroxidases do not play a role in the T. versicolor pulp-bleaching system. Oxidative activity capable of degrading 2-keto-4-methiolbutyric acid (KMB) appeared unique to ligninolytic fungi and always accompanied pulp biobleaching.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S. A new assay for lignin-type peroxidases employing the dye azure B. Appl Environ Microbiol. 1992 Sep;58(9):3110–3116. doi: 10.1128/aem.58.9.3110-3116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982 Apr 1;214(2):452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Archibald F., Roy B. Production of manganic chelates by laccase from the lignin-degrading fungus Trametes (Coriolus) versicolor. Appl Environ Microbiol. 1992 May;58(5):1496–1499. doi: 10.1128/aem.58.5.1496-1499.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M. G. Veratryl alcohol oxidases from the lignin-degrading basidiomycete Pleurotus sajor-caju. Biochem J. 1988 Oct 15;255(2):445–450. doi: 10.1042/bj2550445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheton P. L., Archibald F. S. Manganese complexes and the generation and scavenging of hydroxyl free radicals. Free Radic Biol Med. 1988;5(5-6):325–333. doi: 10.1016/0891-5849(88)90104-9. [DOI] [PubMed] [Google Scholar]
- Clifford D. P., Repine J. E. Measurement of oxidizing radicals by polymorphonuclear leukocytes. Methods Enzymol. 1984;105:393–398. doi: 10.1016/s0076-6879(84)05054-0. [DOI] [PubMed] [Google Scholar]
- Cohen G., Cederbaum A. I. Microsomal metabolism of hydroxyl radical scavenging agents: relationship to the microsomal oxidation of alcohols. Arch Biochem Biophys. 1980 Feb;199(2):438–447. doi: 10.1016/0003-9861(80)90300-8. [DOI] [PubMed] [Google Scholar]
- Cohen G., Heikkila R. E., Allis B., Cabbat F., Dembiec D., MacNamee D., Mytilineou C., Winston B. Destruction of sympathetic nerve terminals by 6-hydroxydopamine: protection by 1-phenyl-3-(2-thiazolyl)-2-thiourea, diethyldithiocarbamate, methimazole, cysteamine, ethanol and n-butanol. J Pharmacol Exp Ther. 1976 Nov;199(2):336–352. [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Tien M., Aust S. D. The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus Phanerochaete chrysosporium. J Biol Chem. 1982 Oct 10;257(19):11455–11462. [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Gold M. H., Kuwahara M., Chiu A. A., Glenn J. K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1984 Nov 1;234(2):353–362. doi: 10.1016/0003-9861(84)90280-7. [DOI] [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Substrate free radicals are intermediates in ligninase catalysis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3708–3712. doi: 10.1073/pnas.83.11.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. L., Reddy C. A. Ethylene production from alpha-oxo-gamma-methylthiobutyric acid is a sensitive measure of ligninolytic activity by Phanerochaete chrysosporium. Biochem J. 1982 Aug 15;206(2):423–425. doi: 10.1042/bj2060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenten R. H., Mann P. J. The oxidation of manganese by peroxidase systems. Biochem J. 1950 Jan;46(1):67–73. doi: 10.1042/bj0460067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Lawrence G. D., Cohen G. In vivo production of ethylene from 2-keto-4-methylthiobutyrate in mice. Biochem Pharmacol. 1985 Sep 15;34(18):3231–3236. doi: 10.1016/0006-2952(85)90339-9. [DOI] [PubMed] [Google Scholar]
- Popp J. L., Kalyanaraman B., Kirk T. K. Lignin peroxidase oxidation of Mn2+ in the presence of veratryl alcohol, malonic or oxalic acid, and oxygen. Biochemistry. 1990 Nov 20;29(46):10475–10480. doi: 10.1021/bi00498a008. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Tang R. H. Ethylene formation from methional. Biochem Biophys Res Commun. 1978 Mar 30;81(2):498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- Sarkanen S., Razal R. A., Piccariello T., Yamamoto E., Lewis N. G. Lignin peroxidase: toward a clarification of its role in vivo. J Biol Chem. 1991 Feb 25;266(6):3636–3643. [PubMed] [Google Scholar]
- Serra M. A., Sabbioni E., Marchesini A., Pintar A., Valoti M. Vanadate as an inhibitor of plant and mammalian peroxidases. Biol Trace Elem Res. 1989;23:151–164. doi: 10.1007/BF02917186. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatadri R., Irvine R. L. Effect of Agitation on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Sep;56(9):2684–2691. doi: 10.1128/aem.56.9.2684-2691.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F. Further studies on ethylene formation from alpha-keto-gamma-methylthiobutyric acid or beta-methylthiopropionaldehyde by peroxidase in the presence of sulfite and oxygen. J Biol Chem. 1969 Aug 25;244(16):4360–4365. [PubMed] [Google Scholar]
- Yang S. F., Ku H. S., Pratt H. K. Photochemical production of ethylene from methionine and its analogues in the presence of flavin mononucleotide. J Biol Chem. 1967 Nov 25;242(22):5274–5280. [PubMed] [Google Scholar]


