Abstract
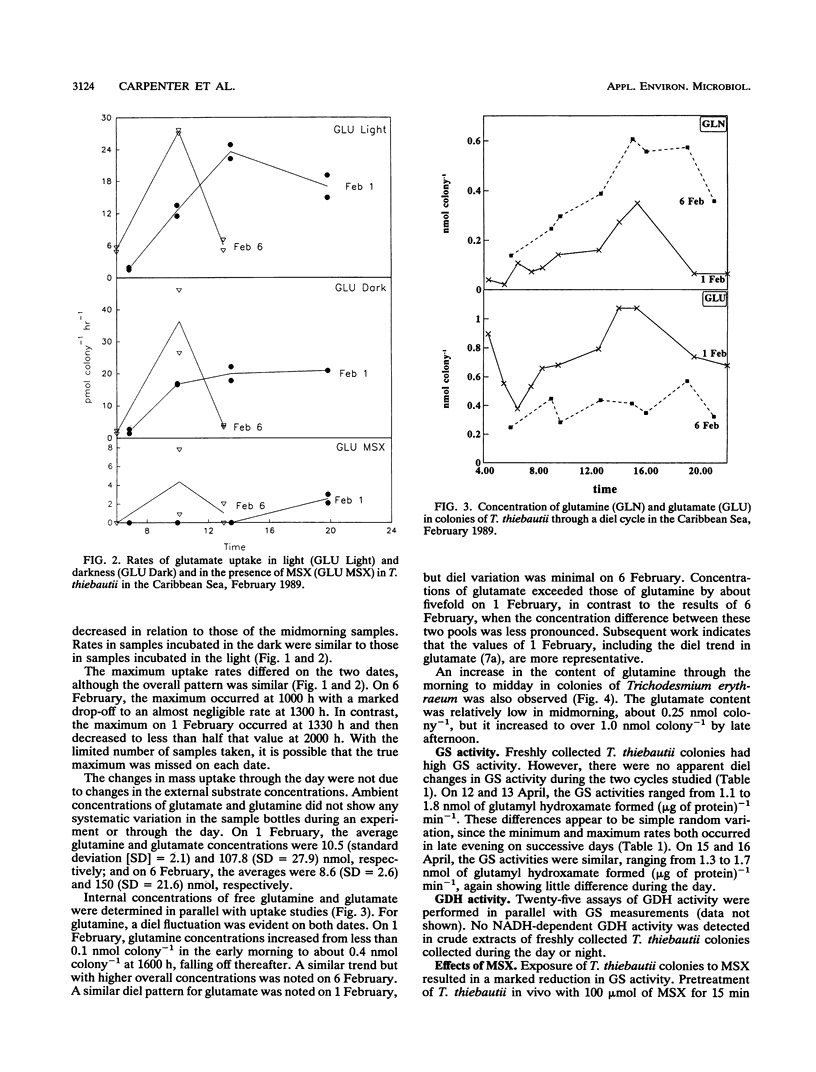
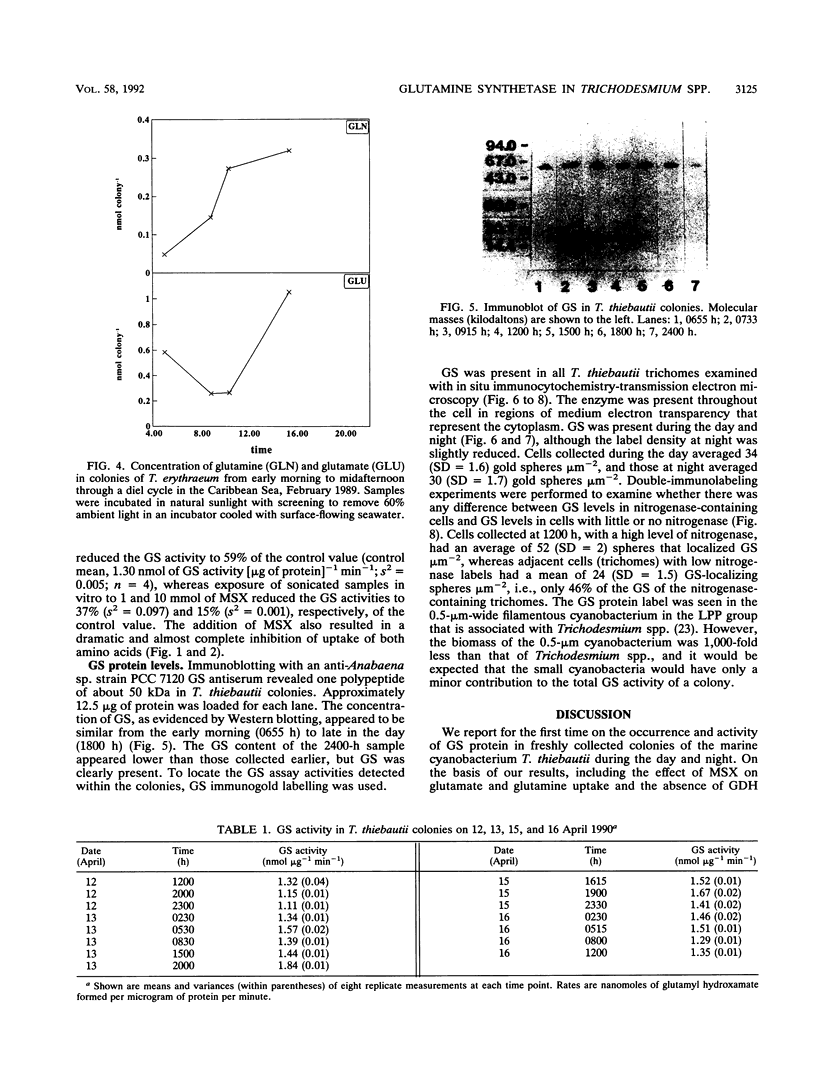
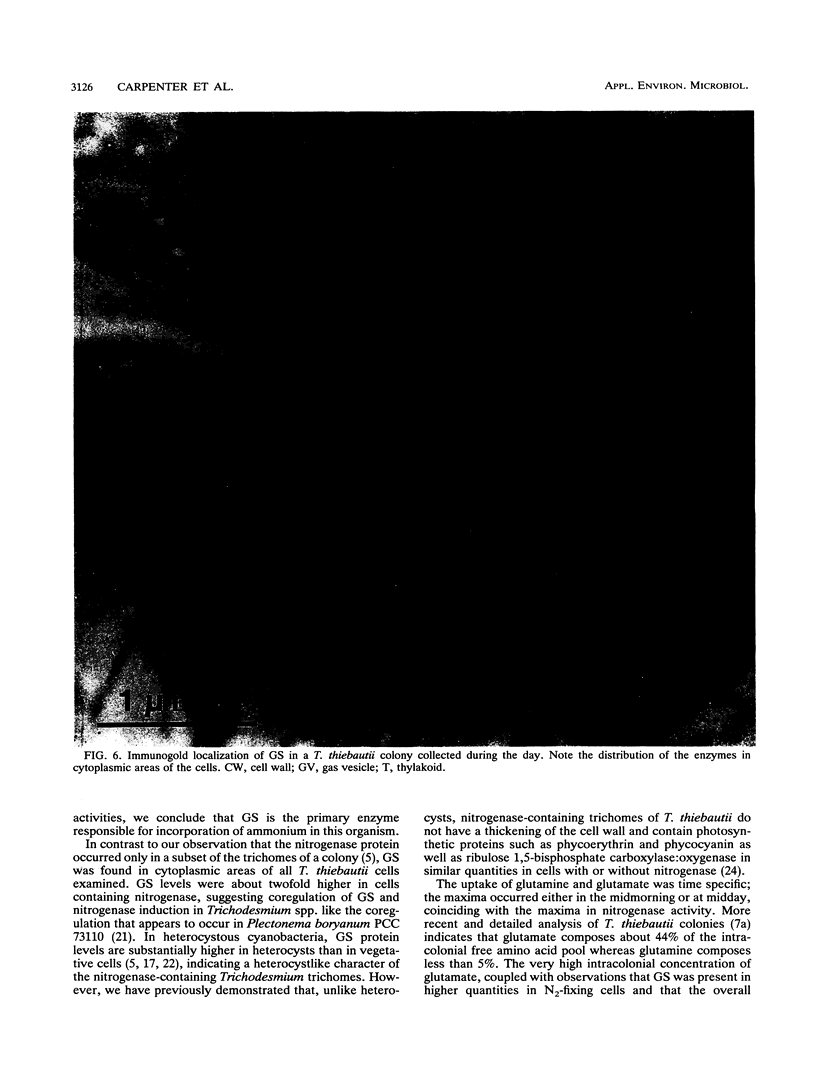
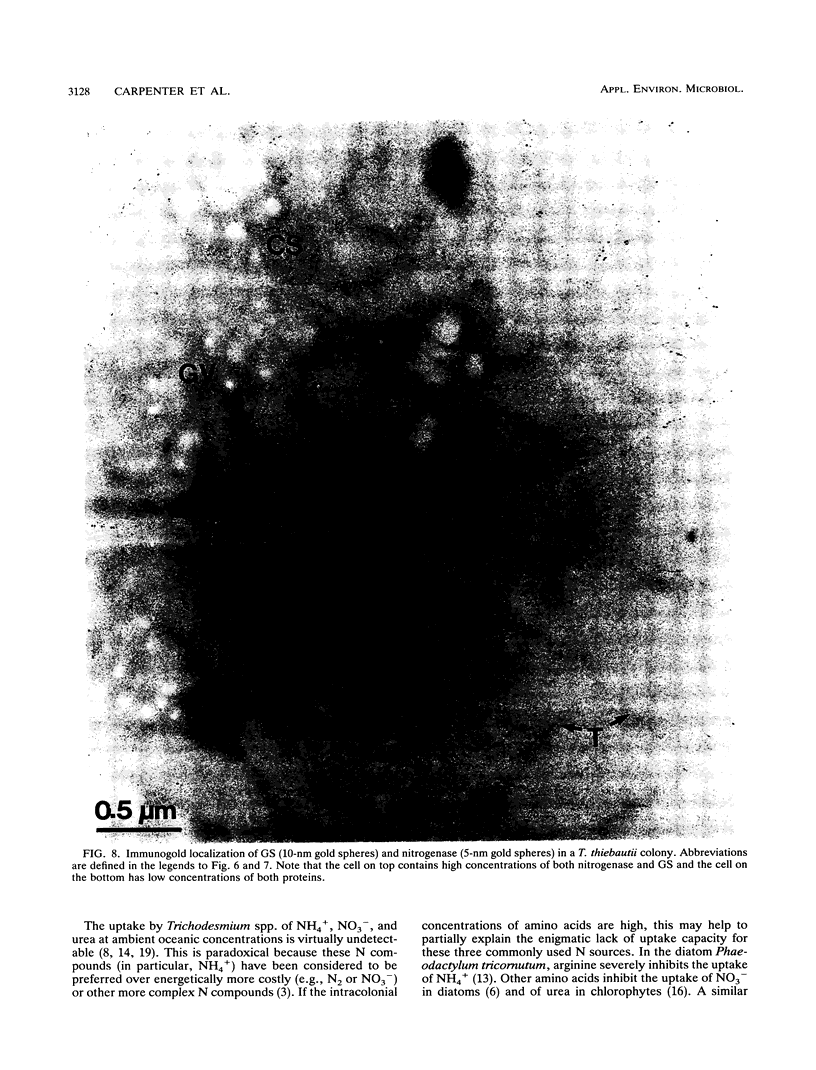
We examined freshly collected samples of the colonial planktonic cyanobacterium Trichodesmium thiebautii to determine the pathways of recently fixed N within and among trichomes. High concentrations of glutamate and glutamine were found in colonies. Glutamate and glutamine uptake rates and concentrations in cells were low in the early morning and increased in the late morning to reach maxima near midday; then uptake and concentration again fell to low values. This pattern followed that previously observed for T. thiebautii nitrogenase activity. Our results suggest that recently fixed nitrogen is incorporated into glutamine in the N2-fixing trichomes and may be passed as glutamate to non-N2-fixing trichomes. The high transport rates and concentrations of glutamate may explain the previously observed absence of appreciable uptake of NH4+, NO3-, or urea by Trichodesmium spp. Immunolocalization, Western blots (immunoblots), and enzymatic assays indicated that glutamine synthetase (GS) was present in all cells during both day and night. GS appeared to be primarily contained in cells of T. thiebautii rather than in associated bacteria or cyanobacteria. Double immunolabeling showed that cells with nitrogenase (Fe protein) contained levels of the GS protein that were twofold higher than those in cells with little or no nitrogenase. GS activity and the uptake of glutamine and glutamate dramatically decreased in the presence of the GS inhibitor methionine sulfoximine. Since no glutamate dehydrogenase activity was detected in this species, GS appears to be the primary enzyme responsible for NH3 incorporation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Hellebust J. A. Transport and Assimilation of Nitrogen by Stichococcus bacillaris Grown in the Presence of Methionine Sulfoximine. Plant Physiol. 1985 Dec;79(4):1125–1126. doi: 10.1104/pp.79.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Capone D. G., O'neil J. M., Zehr J., Carpenter E. J. Basis for Diel Variation in Nitrogenase Activity in the Marine Planktonic Cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1990 Nov;56(11):3532–3536. doi: 10.1128/aem.56.11.3532-3536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H. W., Priscu J. C., Brawner D. L. Immunochemical localization of nitrogenase in marine trichodesmium aggregates: relationship to n(2) fixation potential. Appl Environ Microbiol. 1989 Nov;55(11):2965–2975. doi: 10.1128/aem.55.11.2965-2975.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilbeurgh H., Pettersson G., Bhikabhai R., De Boeck H., Claeyssens M. Studies of the cellulolytic system of Trichoderma reesei QM 9414. Reaction specificity and thermodynamics of interactions of small substrates and ligands with the 1,4-beta-glucan cellobiohydrolase II. Eur J Biochem. 1985 Apr 15;148(2):329–334. doi: 10.1111/j.1432-1033.1985.tb08843.x. [DOI] [PubMed] [Google Scholar]