Abstract
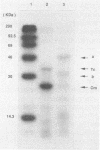
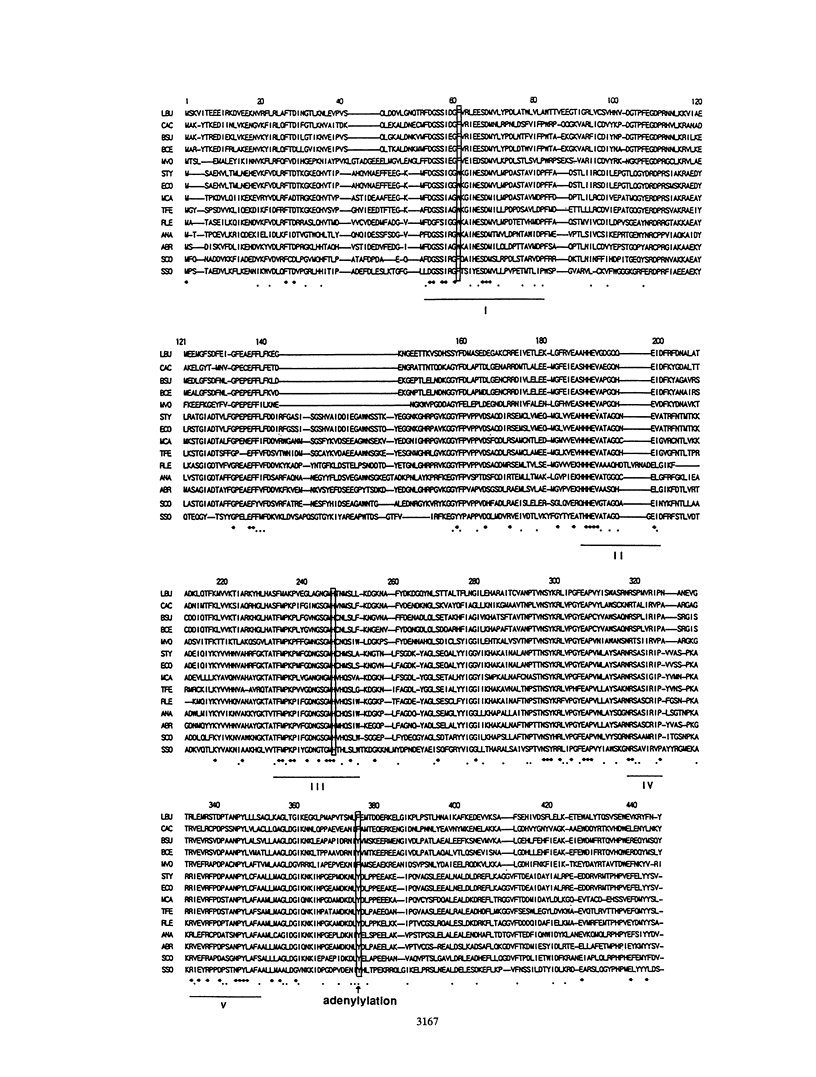
A 3.3-kb BamHI fragment of Lactobacillus delbrueckii subsp. bulgaricus DNA was cloned and sequenced. It complements an Escherichia coli glnA deletion strain and hybridizes strongly to a DNA containing the Bacillus subtilis glnA gene. DNA sequence analysis of the L. delbrueckii subsp. bulgaricus DNA showed it to contain the glnA gene encoding class I glutamine synthetase, as judged by extensive homology with other prokaryotic glnA genes. The sequence suggests that the enzyme encoded in this gene is not controlled by adenylylation. Based on a comparison of glutamine synthetase sequences, L. delbrueckii subsp. bulgaricus is much closer to gram-positive eubacteria, especially Clostridium acetobutylicum, than to gram-negative eubacteria and archaebacteria. The fragment contains another open reading frame encoding a protein of unknown function consisting of 306 amino acids (ORF306), which is also present upstream of glnA of Bacillus cereus. In B. cereus, a repressor gene, glnR, is found between the open reading frame and glnA. Two proteins encoded by the L. delbrueckii subsp. bulgaricus gene were identified by the maxicell method; the sizes of these proteins are consistent with those of the open reading frames of ORF306 and glnA. The lack of a glnR gene in the L. delbrueckii subsp. bulgaricus DNA in this position may indicate a gene rearrangement or a different mechanism of glnA gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozouklian H., Elmerich C. Nucleotide sequence of the Azospirillum brasilense Sp7 glutamine synthetase structural gene. Biochimie. 1986 Oct-Nov;68(10-11):1181–1187. doi: 10.1016/s0300-9084(86)80062-1. [DOI] [PubMed] [Google Scholar]
- Cardy D. L., Murrell J. C. Cloning, sequencing and expression of the glutamine synthetase structural gene (glnA) from the obligate methanotroph Methylococcus capsulatus (Bath). J Gen Microbiol. 1990 Feb;136(2):343–352. doi: 10.1099/00221287-136-2-343. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G., Villafranca J. J. Amino acid sequence of Escherichia coli glutamine synthetase deduced from the DNA nucleotide sequence. J Biol Chem. 1986 Aug 15;261(23):10587–10591. [PubMed] [Google Scholar]
- Colonna-Romano S., Riccio A., Guida M., Defez R., Lamberti A., Iaccarino M., Arnold W., Priefer U., Pühler A. Tight linkage of glnA and a putative regulatory gene in Rhizobium leguminosarum. Nucleic Acids Res. 1987 Mar 11;15(5):1951–1964. doi: 10.1093/nar/15.5.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Levine R. L. Sequence of a peptide susceptible to mixed-function oxidation. Probable cation binding site in glutamine synthetase. J Biol Chem. 1986 Apr 5;261(10):4574–4578. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Rosenkrantz M. S., Sonenshein A. L. Glutamine synthetase gene of Bacillus subtilis. Gene. 1984 Dec;32(3):427–438. doi: 10.1016/0378-1119(84)90018-0. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Kingdon H. S. Primary structure of Escherichia coli glutamine synthetase. II. The complete amino acid sequence of a tryptic heneicosapeptide containing covalently bound adenylic acid. J Biol Chem. 1971 Feb 25;246(4):1099–1106. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hill R. T., Parker J. R., Goodman H. J., Jones D. T., Woods D. R. Molecular analysis of a novel glutamine synthetase of the anaerobe Bacteroides fragilis. J Gen Microbiol. 1989 Dec;135(12):3271–3279. doi: 10.1099/00221287-135-12-3271. [DOI] [PubMed] [Google Scholar]
- Hottinger H., Ohgi T., Zwahlen M. C., Dhamija S., Söll D. Allele-specific complementation of an Escherichia coli leuB mutation by a Lactobacillus bulgaricus tRNA gene. Gene. 1987;60(1):75–83. doi: 10.1016/0378-1119(87)90215-0. [DOI] [PubMed] [Google Scholar]
- Janson C. A., Kayne P. S., Almassy R. J., Grunstein M., Eisenberg D. Sequence of glutamine synthetase from Salmonella typhimurium and implications for the protein structure. Gene. 1986;46(2-3):297–300. doi: 10.1016/0378-1119(86)90415-4. [DOI] [PubMed] [Google Scholar]
- Janssen P. J., Jones W. A., Jones D. T., Woods D. R. Molecular analysis and regulation of the glnA gene of the gram-positive anaerobe Clostridium acetobutylicum. J Bacteriol. 1988 Jan;170(1):400–408. doi: 10.1128/jb.170.1.400-408.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong-Morgenthaler P., Zwahlen M. C., Hottinger H. Lactose metabolism in Lactobacillus bulgaricus: analysis of the primary structure and expression of the genes involved. J Bacteriol. 1991 Mar;173(6):1951–1957. doi: 10.1128/jb.173.6.1951-1957.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J. The ecology and taxonomic status of the lactobacilli. Annu Rev Microbiol. 1976;30:279–301. doi: 10.1146/annurev.mi.30.100176.001431. [DOI] [PubMed] [Google Scholar]
- Nakano Y., Kato C., Tanaka E., Kimura K., Horikoshi K. Nucleotide sequence of the glutamine synthetase gene (glnA) and its upstream region from Bacillus cereus. J Biochem. 1989 Aug;106(2):209–215. doi: 10.1093/oxfordjournals.jbchem.a122834. [DOI] [PubMed] [Google Scholar]
- Nakano Y., Kimura K. Purification and characterization of a repressor for the Bacillus cereus glnRA operon. J Biochem. 1991 Feb;109(2):223–228. [PubMed] [Google Scholar]
- Phillips M. A., Stewart B. E., Qin Q., Chakravarty R., Floyd E. E., Jetten A. M., Rice R. H. Primary structure of keratinocyte transglutaminase. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9333–9337. doi: 10.1073/pnas.87.23.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possot O., Sibold L., Aubert J. P. Nucleotide sequence and expression of the glutamine synthetase structural gene, glnA, of the archaebacterium Methanococcus voltae. Res Microbiol. 1989 Jul-Aug;140(6):355–371. doi: 10.1016/0923-2508(89)90012-0. [DOI] [PubMed] [Google Scholar]
- Rawlings D. E., Jones W. A., O'Neill E. G., Woods D. R. Nucleotide sequence of the glutamine synthetase gene and its controlling region from the acidophilic autotroph Thiobacillus ferrooxidans. Gene. 1987;53(2-3):211–217. doi: 10.1016/0378-1119(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Sanangelantoni A. M., Barbarini D., Di Pasquale G., Cammarano P., Tiboni O. Cloning and nucleotide sequence of an archaebacterial glutamine synthetase gene: phylogenetic implications. Mol Gen Genet. 1990 Apr;221(2):187–194. doi: 10.1007/BF00261719. [DOI] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B. F., Adams R. M., Requadt C., Power S., Mainzer S. E. Expression and nucleotide sequence of the Lactobacillus bulgaricus beta-galactosidase gene cloned in Escherichia coli. J Bacteriol. 1989 Feb;171(2):625–635. doi: 10.1128/jb.171.2.625-635.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Brown S. W., Hirschi K. D., Nomellini J. F., Sonenshein A. L. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J Mol Biol. 1989 Nov 5;210(1):51–63. doi: 10.1016/0022-2836(89)90290-8. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. 5'-adenylyl-O-tyrosine. The novel phosphodiester residue of adenylylated glutamine synthetase from Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3769–3771. [PubMed] [Google Scholar]
- Strauch M. A., Aronson A. I., Brown S. W., Schreier H. J., Sonenhein A. L. Sequence of the Bacillus subtilis glutamine synthetase gene region. Gene. 1988 Nov 30;71(2):257–265. doi: 10.1016/0378-1119(88)90042-x. [DOI] [PubMed] [Google Scholar]
- Wray L. V., Jr, Fisher S. H. Cloning and nucleotide sequence of the Streptomyces coelicolor gene encoding glutamine synthetase. Gene. 1988 Nov 30;71(2):247–256. doi: 10.1016/0378-1119(88)90041-8. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]