Abstract
We demonstrate that adoptive transfer of peritoneal cavity B cells fails to replenish the peripheral B-1 cells in adult B cell-deficient (μ−/−) mice but does replenish adult RAG-1−/− mice. We show that this lack of self-replenishment in μ−/− mice is mediated by strongly inhibitory, radiation-sensitive CD4+ T cells that also function in cotransfer studies to block the reconstitution of B-1 cells and inhibit accumulation of bone marrow-derived B-2 cells in the periphery in irradiated recipients. CD8+ T cells from μ−/− do not mediate this inhibition. The inhibitory CD4+ T cells develop early in life, because B-1 cell replenishment occurs normally when B-1 cells are transferred into μ−/− neonates. Thus, we conclude that the presence of B cells in the neonate conditions the CD4+ T-cell population to permit the establishment and maintenance of normal B cell pools throughout life.
B cell-deficient mice have served widely as tools to assess the contribution of B cells to protective responses against infections with viruses, bacteria, and parasites. By using gene-targeting techniques, mice lacking all peripheral mature B cells have been created by two approaches: the disruption of the gene encoding the membrane-spanning exon of the μ-heavy chain (1) (μ−/−), and the disruption of the JH variable region of the Ig heavy chain (2). Both mutations cause an arrest of B cell development at the proB cell stage because of the inability of B cells to express Ig μ-heavy chains and form functional preB cell receptor complexes. Presumably, the ensuing lack of positive signaling through the membrane preB receptor complex leads to the death of the proB cells (3, 4).
B cell-deficient (μ−/−) mice have been used also as recipients in B cell transfer studies (5, 6). They seem to be ideal recipients for such studies because, in contrast to RAG-1−/− and severe combined immunodeficient mice, they are depleted for B cells but have roughly normal numbers of CD4+ and CD8+ T cells (refs.1 and 2; N. Baumgarth, unpublished results). In principle, they should have adequate biological “space” for B cells in the periphery and should be readily reconstituted by B cell sources. In the past, selective depletion of B cells has been achieved by chronic administration of anti-IgM antibody (7). Transfers of allotype-mismatched (IgM+ CD5+) B-1 cells shortly after birth into allotype-specific anti-IgM antibody-treated mice demonstrated that B-1 cells can expand and replenish the B-1 cell compartments of peritoneal cavity and spleen for the life of the animal (8–14). Antibody-treatment before cell transfer is necessary to deplete all host B-1 cells, because these cells act via a not yet fully defined negative feedback mechanism to inhibit replenishment of the recipient with the donor B-1 cells (12). Thus, because transfers of B-1 cells into B cell-deficient animals made in such a way always fully and permanently reconstitute B-1 cells (8–14), there is strong reason to expect that transfers of adult peritoneal B-1 cells to μ−/− mice would also result in the complete and permanent reconstitution of B-1 cells in the recipients.
Surprisingly, therefore, we report in this study that the transfer of B-1 cells into adult μ−/− mice does not result in reconstitution of the B-1 cell compartment. Furthermore, we show that this reconstitution failure is because of the presence of strongly inhibitory CD4+ T cells that not only inhibit B-1 cell reconstitution, but also block the accumulation of long-lived recirculating mature B-2 cells. Finally, we show that transfer of B-1 cells to neonatal, rather than adult, μ−/− mice successfully reconstitutes the B-1 cell compartments and thus conclude that the presence of B cells during the neonatal period enables the development of a normal adult T-cell population that permits B cell maturation to occur.
Materials and Methods
Mice and Adoptive Cell Transfers.
B6.C20 [a-allotype (Igha)], C57BL/6 (Ighb), and congenic μ−/− mice (The Jackson Laboratory), originally described in ref. 1 were bred and maintained at the Animal Facility at Stanford University. Nonirradiated recipients received intravenously 5 × 106 peritoneal-cavity cells (PerC) in 200 μl of PBS from 2-month-old C57/Igha mice. Lethally and sublethally irradiated mice (850 and 400 rad, respectively) received intravenously 5 × 106 PerC from C57/Igha and 3 × 106 bone marrow cells from C57BL/6. In some experiments, recipients received in addition 2 × 106 fluorescent-activated cell sorter (FACS)-purified lymph node CD4+ or CD8+ T cells from μ−/− or C57BL/6 mice. Preliminary experiments established that the μ−/− mice were fully congenic to the C57BL/6 mice from our colony. No graft-versus-host or host-versus-graft disease was observed in the more than 200 cell transfer experiments performed and no animals died after cell transfers. In addition, in vitro cocultures of splenic cells from μ−/− mice with either C57BL/6 or C57BL/6.Igha (B6.C20, referred to here as C57/Igha) splenic cells did not lead to spontaneous proliferation (data not shown).
Cell Preparation.
Single-cell suspensions of spleens and lymph nodes were prepared according to standard methods and erythrocytes were lysed with 0.14 M NH4Cl/20 mM Tris (pH 7.4). Single-cell suspensions of PerC and bone marrow from the femur and tibia were prepared by flushing peritoneal cavity and bone marrow cavities with staining medium (biotin- and flavin-deficient RPMI medium 1640, supplemented with 4% newborn calf serum).
Ten-Color FACS Analysis and FACS Purification.
Single-cell suspensions from spleens and PerC were stained simultaneously with antibody conjugates specific to: CD21-fluorescein (FITC) (mAb 7G6) (PharMingen, San Diego, CA); CD43 phycoerythrin (PE) (S7); CD22 biotin (Cy34.1); CD5 biotin (53–7.3); CD23 Texas Red (TR) (B3B4); CD11b allophycocyanin (APC) (M1/70); IgM Cy7-APC (331); IgMa Cy7-APC (DS-1); IgMb Cy7-APC (AF6.78); IgD Cy7-PE (1126); IgDa Cy7-PE (AMS9); IgDb Cy7-PE (AF6.122); B220 Cascade Blue (RA3–6B2); CD4 Cascade Yellow or Cascade Blue (GK1.5); CD8 Cascade Yellow or Cascade Blue (53.6.7); macrophage Cascade Yellow or Cascade Blue (F4/80). Streptavidin-Cy5-PE was used as a second-step reagent. Noncommercial conjugates and tandem dyes were prepared as outlined (15–17). The novel fluorochrome Cy5.5-APC was prepared and conjugated to the anti-CD19 mAb similar to the method described for Cy7-APC (17). Propidium iodide was added at 0.25 μg/ml. Cells were assayed with a modified triple laser Cytomation/Becton Dickinson hybrid FACS, described in refs. 18 and 19, and data were analyzed with the flowjo software (Treestar, San Carlos, CA).
For FACS-sort of CD3+, CD4+, and CD8+ lymph node cells, cell suspensions were stained with anti-CD3 Cy5-PE, anti-CD4 FITC, and anti-CD8 PE and sorted on a FACStar Plus (Becton Dickinson) instrument. Cells were reanalyzed immediately after sort and purities were >96%.
ELISA.
The serum concentrations of a- and b-allotype IgM were determined as described (14).
Statistical Analysis.
Statistical significance was tested with the nonparametric Wilcoxon/Kruskal Wallis Rank Test, or when appropriate, with the two-tailed Student's t test.
Results
Lack of B-1 Cell Self-Replenishment after Transfer into Adult μ−/− Mice.
μ−/−mice completely lack peripheral B cells and should be a suitable recipient for B-1 cell replenishment after adoptive cell transfer, because the only factor known to inhibit their expansion is the presence of host-derived B-1 cells (12). We therefore tested for B-1 cell reconstitution in μ−/− mice after adoptive transfer of PerC from IgH allotype congeneic (C57BL/6.Igha) mice. As controls, we used nonirradiated adult congenic RAG-1−/− mice which lack mature B and T cells, and nonirradiated 2-day-old congenic C57BL/6 (Ighb) mice, treated from birth for 6 weeks with anti-IgMb to interrupt all recipient B cell development for the duration of treatment (13). Because of the allotype specificity of the anti-IgMb treatment, donor-derived B cells are not affected by the treatment.
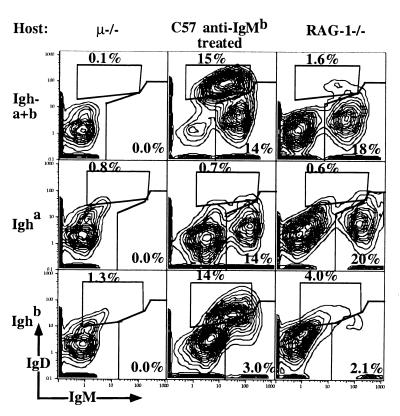
Surprisingly, 2 months after cell transfer of FACS-sorted peritoneal B-1 cells to μ−/− recipients, B-1 cells from the (Igha) PerC donor were completely absent from the recipient peritoneal cavity (Fig. 1 and Table 1) and spleen (data not shown). In contrast, PerC donor-derived B-1 cells (Igha) were present at roughly normal levels in the peritoneal cavity and spleen of RAG-1−/− recipients and in nonirradiated 2-day-old C57BL/6 (Ighb) recipients treated from birth for 6 weeks with anti-IgMb. Consistent with these FACS data, μ−/− recipients lacked serum IgM, whereas the PerC donor-derived serum IgM levels in the other recipients were comparable to the levels of control C57/Igha mice (Table 1). Therefore, B-1 cells can neither establish a self-replenishing B cell population nor secrete IgM after transfer into adult μ−/− mice.
Figure 1.
Lack of B-1 cell replenishment after PerC transfer into adult μ−/− recipients. PerC from C57/Igha donors was transferred into adult μ−/− mice, C57BL/6 neonates treated from birth for two month with anti-IgMb, and into adult RAG-1−/− mice. 5% contour plots of recipient PerC cells are shown, stained for total (Igha + Ighb) and allotype-specific IgM and IgD of live, nonT cells/nonmacrophages (Fig. 3B). B-1 cells were identified as IgMhi IgDlo and B-2 cells as IgMlo IgDhi. Additional staining for CD5, CD11b, and CD23 confirmed their belonging to the different lineages (not shown). Data are summarized in Table 1.
Table 1.
B cell reconstitution after PerC transfer to nonirradiated hosts
| Host | n | Recipient Igh | PerC§ Igh | % B-2* ± SD | % B-1* ± SD | Serum IgMa, μg/ml | Serum IgMb, μg/ml |
|---|---|---|---|---|---|---|---|
| μ−/− | 8 | Ighb | Igha | 0.3 ± 0.3 | 0.03 ± 0.02 | < 0.015 | < 0.015 |
| C57-Ab† | 7 | Ighb | Igha | 11 ± 2.6 | 18 ± 2.4 | 250 ± 48 | 330 ± 59 |
| RAG−/−‡ | 5 | Ighb | Igha | 3.6 ± 2.4 | 19 ± 5.4 | 230 ± 89 | 540 ± 440 |
| C57/Igha | 6 | Igha | None | 22 ± 2.4 | 26 ± 3.7 | 210 ± 130 | < 0.015 |
| C57BL/6 | 7 | Ighb | None | 13 ± 5.2 | 22 ± 5.5 | < 0.015 | 590 ± 380 |
*Shown is the frequency (± SD) of cells among total live recipient PerC cells.
†Mice were treated from birth for 6 weeks with a total of 2 mg per mouse anti-IgMb.
‡Mice received i.v. 3 × 106 bone marrow from C57BL/6 2–3 months before analysis.
§Mice received 5 × 106 PerC cells from C57/Igha 2–3 months before analysis.
Restoration of B-1 Cell Self-Replenishment in Adult μ−/− Mice.
Next we determined whether B-1 cell reconstitution occurs after irradiation of adult μ−/− mice and whether the source of the bone marrow cells injected simultaneously with the PerC influences B-1 cell reconstitution. In contrast to the data obtained with nonirradiated mice, PerC donor-derived B-1 cell reconstitution occurred in the spleen and PerC of all lethally (Fig. 2) and sublethally (data not shown) irradiated adult recipients, including μ−/− mice. Thus, irradiation is sufficient to enable long-term reconstitution of B-1 cells in adult μ−/− mice.
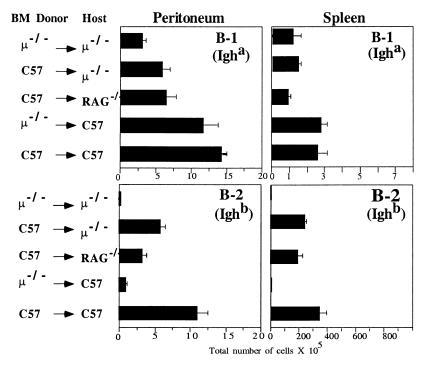
Figure 2.
B-1 cell replenishment occurs in irradiated adult μ−/− mice after adoptive cell transfer. PerC (5 × 106) from C57/Igha donors and 3 × 106 bone marrow cells from bone marrow (BM) donors were transferred into irradiated recipients as indicated. Shown are means (± SE) of total B-1 and B-2 cells recovered from groups of recipient mice (group sizes shown in Table 2) 2–3 months after cell transfer. Identification of B-1 and B-2 cells was done by FACS (Fig. 3A).
B-1 cell reconstitution was not affected by the bone marrow source in any of the recipients. Regardless whether the B-1 cells were cotransferred with bone marrow from C57BL/6 or μ−/− mice, the numbers of PerC donor-derived B-1 cells were comparable in peritoneal cavity and spleen in irradiated C57BL/6 recipients. The type of recipient, in contrast, did influence B-1 reconstitution in that the number of PerC donor-derived B-1 cells was significantly lower (P < 0.001) in μ−/− and RAG-1−/− recipients than in C57BL/6 recipients (Fig. 2). Interestingly, reconstitution of the spleen and peritoneal cavity of these recipients with bone marrow-derived (Ighb) B cells was similarly influenced (Fig. 2). Consistent with reconstitution data observed by FACS, PerC donor-derived serum IgMa was present in all recipients, including μ−/− mice (Table 2). Thus, the inhibition of B-1 cell replenishment in the μ−/− mice is caused by a mechanism that can be overcome in most part by irradiation and is not mediated by cells present in μ−/− bone marrow.
Table 2.
Serum IgM levels after PerC and bone marrow transfer into irradiated hosts
| Host* | n | Donor BM | Donor PerC | Serum IgMa†, μg/ml ± SE | Serum IgMb†, μg/ml ± SE | IgMa + IgMb‡, μg/ml |
|---|---|---|---|---|---|---|
| μ−/− | 7 | μ−/− | C57/Igha | 340 ± 170 | < 0.015 | 340 |
| μ−/− | 7 | C57 | C57/Igha | 600 ± 140 | 510 ± 98 | 1,100 |
| RAG1−/− | 5 | C57 | C57/Igha | 370 ± 68 | 710 ± 44 | 1,100 |
| C57 | 4 | μ−/− | C57/Igha | 1,100 ± 91 | 160 ± 7.0 | 1,200 |
| C57 | 10 | C57 | C57/Igha | 570 ± 23 | 240 ± 24 | 820 |
Lethally irradiated recipients before transfer of 5 × 106 PerC and 3 × 106 bone marrow cells (BM) from donors indicated in Table. Sera were taken from mice 2–3 months after transfer and analyzed by ELISA.
†Data shown are mean titers ± standard error.
‡Mean total IgM levels were calculated by adding the values obtained by ELISA for IgMa and IgMb.
T-Cell Cotransfer Studies to Test for T-Cell Influence on B-1 Reconstitution.
Because we found that B-1 cell self-replenishment occurs in the B- and T-cell-deficient RAG−/− mice (Fig. 1) and the block in μ−/− mice is overcome by irradiation, we next tested whether the presence of T cells inhibits B cell reconstitution in μ−/− mice. For this study, we cotransferred 2 × 106 FACS-sorted CD4+ or CD8+ lymph node T cells from either C57BL/6 or μ−/− mice (both Ighb) with congenic Igha PerC and C57BL/6 bone marrow cells, as a source for hematopoietic stem cells, into lethally irradiated μ−/− (Fig. 3) or C57BL/6 mice (data not shown). Transfers into both types of recipients yielded similar results.
Figure 3.
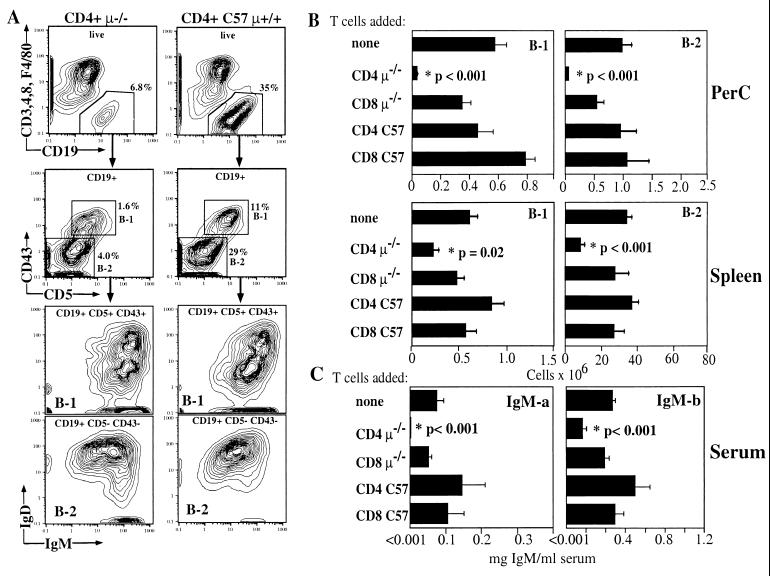
(A) FACS analysis of PerC isolated from irradiated μ−/− recipients 2 months after cotransfer of 2 × 106 FACS-purified CD4+ T cells from wild-type (CD4+ C57 μ+/+) or μ−/− mice (CD4+ μ−/−), 5 × 106 PerC cells from C57/Igha and 3 × 106 bone marrow cells from C57BL/6 (Ighb) mice. Contour plots (5%) are shown. The relative frequency of total live CD19+, CD3−, CD4−, CD8−, and F4/80− and IgDhi IgMlo follicular B-2 cells and IgDlo IgMhi CD5+ and CD43+ B-1 cells is indicated. (B) Summary of data from three independent transfer experiments with five groups (n = 6–10 per group) of recipient μ−/− mice that had received PerC (Igha) and bone marrow (Ighb) together with FACS-purified CD4+ or CD8+ T cells from either wild-type C57BL/6 or μ−/− mice, or no T cells. Total numbers (± SE) of the indicated B cell subpopulations in the peritoneal cavity and spleen are shown, calculated from the relative cell numbers obtained by FACS and total live cell counts obtained by counting acridine orange/ethidium bromide-stained cells. (C) Serum levels of PerC donor (B-1)-derived IgM-a and bone marrow-derived IgM-b (means ± SE) in the five groups of recipient mice determined by ELISA.
Fig. 3A illustrates the FACS analysis strategy used to determine the influence of cotransferred T cells on B cell replenishment in PerC. B-1 and B-2 cells were identified by their differential expression of CD5 and CD43 and the difference in their levels of IgM and IgD expression. Additional staining for CD23, CD11b (MAC-1), B220, and allotype-specific staining for IgM and IgD confirmed that these cells were derived from the PerC and bone marrow donor, respectively (data not shown). In the spleen, similar staining was performed including anti-CD21 staining to better distinguish B-1 cells from marginal zone B cells (not shown).
CD4+ T Cells from μ−/− Mice Inhibit B-1 Cell Reconstitution and IgM Production.
Data summarized from three independent cell-transfer experiments demonstrate that cotransfer of PerC and bone marrow with CD4+ T cells from μ−/− mice dramatically decreases the numbers of B-1 cells in both peritoneal cavity and spleen of recipient mice (Fig. 3 A and B). Moreover, no PerC donor-derived serum IgMa was detected in these recipients (Fig. 3C). This is in contrast to mice that received the same B cell sources but did not receive T cells, mice that received CD4+ or CD8+ T cells from C57BL/6 mice, or mice that received CD8+ T cells from μ−/− mice. In all of these recipients, substantial numbers of B-1 cells were found both in the peritoneal cavity and the spleen, and sera from all mice had substantial levels of serum IgMa (Fig. 3C). Thus, we conclude that the CD4 T cells from μ−/− mice mediate the inhibition of B-1 cell development in the transfer recipient.
CD4+ T Cells from μ−/− Mice Inhibit B-2 Cell Reconstitution and IgM Production.
Importantly, the cotransfer of μ−/− CD4+ T cells also inhibited reconstitution of peritoneal cavity and splenic follicular B-2 cells (Fig. 3). The most dramatic effects were seen in the peritoneal cavity of these mice, where similar to the B-1 cells, very few B-2 cells were found. Splenic follicular B cells were recovered, albeit at greatly reduced levels. Serum IgMb (bone marrow donor derived) levels were also significantly reduced in mice that received CD4+ T cells from μ−/− mice (Fig. 3C). As in the B-1 cell reconstitution data, mice that did not receive CD4+ T cells from μ−/− mice showed no significant decrease in B-2 cell reconstitution or serum IgMb levels. Taken together, data from these cell-transfer experiments show that CD4+ but not CD8+ T cells from μ−/− mice strongly inhibit the reconstitution and function of PerC- and bone marrow-derived mature B cells even 2 months after transfer.
B-1 Cell Replenishment Is μ−/− Recipient Age Dependent.
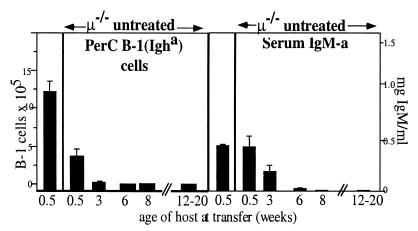
Because B-1 cell reconstitution does not occur when B-1 cells are transferred into adult μ−/− mice but does occur when B-1 cells are transferred to neonatal mice rendered B cell deficient by anti-IgMb treatment (Fig. 1), we determined next whether B-1 cells are reconstituted by transfer of adult B-1 cells to young μ−/− mice. In essence, we found that the numbers of PerC donor-derived B-1 cells present in recipient μ−/− mice 8 weeks or more after transfer and the levels of PerC donor-derived serum IgMa are inversely related to the age of the recipient at the time of cell transfer (Fig. 4). When the PerC cell transfer was done within a few days after birth, substantial numbers of B-1 cells were found in μ−/− mice 2–3 months after transfer. The number of B-1 cells found in these μ−/− mice was somewhat lower than observed in anti-IgMb-treated C57BL/6 mice; however, their B-1 cell-derived serum IgM levels were comparable. No B-1 cells or serum IgM were found in any PerC transfers done with recipient μ−/− mice at age 8 weeks or older. Thus, inhibitory CD4+ T cells in μ−/− are not present at birth and emerge slowly as the animal develops, but only in the absence of mature B cells.
Figure 4.
Lack of B-1 cell replenishment in μ−/− mice is recipient age dependent. Groups of recipient μ−/− mice varying in age as indicated received 5 × 106 PerC cells from C57/Igha donors. Control C57BL/6 neonates (first column) received PerC and were treated for 6 weeks with anti-IgMb. PerC was analyzed for the presence of donor-derived B-1 cells by FACS (Fig.3A). Means ± SE of B-1 cells recovered 2–3 months after cell transfer or end of antibody treatment are shown. Serum IgMa levels (means ± SE) were measured by ELISA.
Discussion
Early studies using chronic treatment with anti-IgM to deplete B cells, and two recent studies with μ−/− mice, collectively support the notion that the presence of B cells is necessary for the normal functional development of T cells (20–27). The earlier work suggests that certain T cells necessary for antibody production are absent from anti-IgM-treated mice, whereas the two most recent studies demonstrate the inability of T cells from μ−/− mice to produce normal levels of various cytokines in response to infection with lymphocytic choriomeningitis virus (21) and to protein immunization (20). The data presented here demonstrate that a CD4+ T-cell population in adult μ−/− mice is capable of inhibiting the development of all mature B cell pools. In cotransfer studies, CD4+ but not CD8+ T cells from these mice act to block both B-2 and B-1 cell reconstitution even 2 months after transfer whereas similarly cotransferred CD4+ T cells from normal mice do not impair B cell accumulation. The presence of these T cells in adult μ−/− mice is sufficient to explain the failure of transferred B-1 cells to reconstitute the B-1 population in the μ−/− mice
Our data also demonstrate that the ability of T cells to allow replenishment of the peripheral B cell pools is acquired during T-cell development. Transfer of B-1 cells into 3-day-old μ−/− mice leads to B-1 cell replenishment, whereas B-1 cell transfer only 3 weeks later results in very little replenishment (Fig. 4). Thus, the first 3 weeks of life, during which phenotypically normal adult populations of CD4+ and CD8+ αβ T cells develop, also emerges as a crucial period during which the T-cell compartment is conditioned to permit normal B cell development. In essence, the absence of B cells in μ−/− mice during this period results in the development of an abnormal T-cell population that dramatically impairs development of all mature B cell pools in adoptive recipients.
These findings suggest that B-1 cells may have an important role to play during ontogeny. B-1 cells are present early in development, before the first mature thymus-derived T cells appear in the periphery (9, 28, 29). Furthermore, although B-1 cells are rarely found in lymph nodes, they are present in the thymus of newborn and adult mice (30). Thus, by acting to ensure normal functional T-cell development during the neonatal period, before the first wave of bone marrow-derived B cells accumulate in the periphery, B-1 cells enable normal antibody production by their own progeny and by B-2 cells that provide the bulk of the adaptive immune response (14).
The close proximity of B-1 cells and the developing T-cell populations in the neonate could reflect the need for education of T-cell populations to support B cell development in adults. However, T cells are not required for B cell maturation, because B cells develop in RAG-1−/− mice in the complete absence of T cells (Figs. 1 and 2), and mice lacking αβ T cells have normal or even higher numbers of all peripheral B cells (31). Therefore, we conclude that CD4+ T cells from μ−/− mice actively inhibit the expansion of bone marrow and PerC-derived mature B cells rather than fail to provide positive signals to enable B cell development. Consequently, we view the role of B-1 cells during the neonatal period as revolving around the depletion of or functional alteration of T cells that would otherwise persist and inhibit the accumulation of B cells in the adult.
Consistent with T cells in adult mice being responsible for inhibition of B cell development, transferred B-1 cells readily reconstitute the B-1 compartments in μ−/− mice when the vast majority of peripheral T cells are removed by the irradiation before cell transfer (Fig. 2). This reconstitution occurs even when the irradiated μ−/− mice are rescued with μ−/− bone marrow.
At least three different scenarios can be envisaged for the way in which CD4+ T cells in μ−/− mice inhibit the accumulation of mature B cells in the peripheral lymphoid compartments. First, T cells that develop in the absence of B cells and Ig might recognize the B cells as “non self” and remove these cells directly via cytolysis. Second, CD4+ T cells could activate CD8+ T cells or other cells, which could then give an inhibitory or death signal to the B cells. Third, direct CD4+ T cell–B cell interaction could occur between B-1 cells and T cells, e.g., CD4+ T cells from μ−/− mice might deliver an inhibitory or death signal to the cells so that no mature B cells accumulate in the periphery. The CD95/CD95L pathway, which is known to play a role during cognate CD4+ T cell–B cell interaction (32, 33), is a possible death pathway for such a direct interaction.
Although all of these mechanisms are reasonable, we find no evidence for nonself recognition of B cells by μ−/− T cells. Because early B cell precursors are present in the μ−/− mice, the T cells in these mice should be tolerant to all B cell proteins that are expressed until the proB cell stage. The expression and recognition of novel self-antigens such as the pre-B cell receptor would be expected to lead to a deletion of B cells at an early stage of development; however, we find mature B cells in the periphery, although in greatly reduced numbers, and we find relatively normal numbers of immature B cells (N.B., G.C.J., O.C.H., and L.A.H., unpublished work). Second, B cell deletion because of nonself recognition would be expected to be mediated also by cytolytic CD8+ T cells, because B cells continuously express self-antigens via their MHC class I molecules; however, the inhibition of B cell development that we observe is mediated by cotransferred CD4+ T cells, and not by cotransferred CD8+ T cells. Finally, we have found that coculturing T cells from μ−/− mice together with total splenic B cells does not induce T-cell proliferation, even when μ−/− mice are primed with B cells before the T cells are isolated from their lymph nodes (N.B., unpublished work). Therefore, if CD4+ T cells recognize the B cells as nonself and induce cytolyis in vivo, this would have to occur indirectly via activation of a cell population other then CD8+ T cells.
As we will show elsewhere (N.B., G.C.J., O.C.H., and L.A.H., unpublished work), the CD4+ T cells from μ−/− mice inhibit B cell differentiation at or shortly after the immature/transitional stage leading to the lack of follicular B cells shown in this study (Fig. 3). At this stage, immature B cells accumulate in the T-cell-rich areas of the spleen periarteriolar lymphoid sheath (PALS) (34–36), where a small fraction of B cells is then selected into the long-lived mature follicular B cell pool or perhaps become marginal zone B cells (3, 4, 37). The migration of immature B cells to the T-cell areas suggests that T-cell–B cell interaction at this point is a physiological process. Moreover, interaction of immature B cells with CD4+ T cells in the PALS has been demonstrated in a number of transgenic models (3, 4, 32, 38–43). The third scenario, i.e., the physiological interaction of CD4+ T cells with immature B cells, seems therefore most consistent with our data.
Immature B cells are exquisitely sensitive to induction of apoptosis (43, 44), but can be rescued by provision of IL-4 or signaling through CD40 (45) by CD4+ T cells. Data demonstrating that μ−/− T cells produce less IL-4 after stimulation (20) are not sufficient, however, to explain the inhibitory effect of the CD4+ T-cell population from μ−/− mice. μ−/− CD4+ T cells inhibit B cell maturation in the presence of normal mature T cells derived from cotransferred PerC and bone marrow, that should be able to provide the required signals. Furthermore, T cells are not required for B cell maturation, because B-1 cells develop in RAG-1−/− mice in the complete absence of T cells (Figs. 1 and 2), and mice lacking αβ T cells have normal or even higher numbers of all peripheral B cells (31). Therefore, we conclude that CD4+ T cells from μ−/− mice actively inhibit the expansion of bone marrow and PerC-derived mature B cells rather than lack the ability to provide a positive signal(s).
In conclusion, our findings have important implications for both T- and B cell development. In essence, they demonstrate that the presence of B-1 cells early in ontogeny might be a necessary first step to enable the conditioning of the developing CD4+ T cells, so that these T cells can permit or even regulate the size of the peripheral B cell pools.
Acknowledgments
We thank the FACS Development Group at Stanford University, in particular Drs. D. Parks, R. Stovel, and M. Bigos, for their work on establishing 10-color FACS. We thank Drs. A. Fell, M. Roederer, D. Tarlinton, and B. Devlin for their comments on drafts of this manuscript. This work was supported by Grant AI-34762–34 from the National Institutes of Health to L.A.H.
Abbreviations
- PerC
peritoneal-cavity cells
- APC
allophycocyanin
- PE
phycoerythrin
- FACS
fluorescent-activated cell sorter
References
- 1.Kitamura D, Roes J, Kuehn R, Rajewsky K. Nature (London) 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Trounstine M, Alt F W, Young F, Kurahara C, Loring J F, Huszar D. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 3.Melchers F, Rolink A, Grawunder U, Windkler T H, Karasuyama H, Ghia P, Andersson J. Curr Opin Immunol. 1995;7:214–227. doi: 10.1016/0952-7915(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 4.Goodnow C C, Cyster J G, Hartley S B, Bell S E, Cooke M P, Healy J I, Akkaraju S, Rathmell J C, Pogue S L, Shokat K P. Adv Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 5.Agenes F, Freitas A A. J Exp Med. 1999;189:319–330. doi: 10.1084/jem.189.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth R, Mamula M J. J Exp Biol. 1997;200:2057–2062. doi: 10.1242/jeb.200.14.2057. [DOI] [PubMed] [Google Scholar]
- 7.Cooper M D, Kearney J F, Gathings W E, Lawton A R. Immunol Rev. 1980;52:29–53. doi: 10.1111/j.1600-065x.1980.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 8.Riggs J E, Stowers R S, Mosier D E. J Exp Med. 1990;172:475–485. doi: 10.1084/jem.172.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantor A B, Herzenberg L A. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 10.Kantor A B, Stall A M, Adams S, Watanabe K, Herzenberg L A. Int Immunol. 1995;7:55–68. doi: 10.1093/intimm/7.1.55. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa K, Hardy R R, Herzenberg L A, Herzenberg L A. J Exp Med. 1985;161:1554–1565. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalor P A, Herzenberg L A, Adams S, Stall A M. Eur J Immunol. 1989;19:507–513. doi: 10.1002/eji.1830190315. [DOI] [PubMed] [Google Scholar]
- 13.Lalor P A, Stall A M, Adams S, Herzenberg L A. Eur J Immunol. 1989;19:501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- 14.Baumgarth N, Herman O C, Jager G C, Brown L, Herzenberg L A. Proc Natl Acad Sci USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy R R. In: Handbook of Experimental Immunology. Weir D M, editor. Vol. 1. Palo Alto: Blackwell Scientific; 1986. pp. 13.1–13.9. [Google Scholar]
- 16.Anderson M T, Baumgarth N, Haugland R P, Gerstein R M, Tjioe I, Herzenberg L A, Herzenberg L A. Cytometry. 1998;33:435–444. doi: 10.1002/(sici)1097-0320(19981201)33:4<435::aid-cyto7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Roederer M, Kantor A B, Parks D R, Herzenberg L A. Cytometry. 1996;24:191–197. doi: 10.1002/(SICI)1097-0320(19960701)24:3<191::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Roederer M, DeRosa S, Gerstein R, Anderson M, Bigos M, Stovel R, Nozaki T, Parks D, Herzenberg L, Herzenberg L. Cytometry. 1997;29:1–12. doi: 10.1002/(sici)1097-0320(19971201)29:4<328::aid-cyto10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Bigos M, Baumgarth N, Jager G C, Herman O C, Nozaki T, Stovel R T, Parks D R, Herzenberg L A. Cytometry. 1999;36:36–45. doi: 10.1002/(sici)1097-0320(19990501)36:1<36::aid-cyto5>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Macaulay A E, DeKruyff R H, Umetsu D T. J Immunol. 1998;160:1694–1700. [PubMed] [Google Scholar]
- 21.Homann D, Tishon A, Berger D P, Weigle W O, Von Herrath M G, Oldstone M B A. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HayGlass K T, Naides S J, Benacerraf B, Sy M-S. J Mol Cell Immunol. 1985;2:107–117. [PubMed] [Google Scholar]
- 23.Janeway C A, Jr, Tite J P, Bottomly K, Flood P. J Mol Cell Immunol. 1985;2:118–120. [PubMed] [Google Scholar]
- 24.Flood P M, Janeway C A, Jr, Gershon R K. J Mol Cell Immunol. 1984;1:167–176. [PubMed] [Google Scholar]
- 25.Kim K J, Rollwagen F, Asofsky R, Lefkovits I. Eur J Immunol. 1984;14:476–482. doi: 10.1002/eji.1830140517. [DOI] [PubMed] [Google Scholar]
- 26.Sy M-S, Lowy A, HayGlass K, Janeway C A, Jr, Gurish M, Greene M I, Benacerraf B. Proc Natl Acad Sci USA. 1984;81:3846–3850. doi: 10.1073/pnas.81.12.3846. .C. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sy M-S, Benacerraf B. Immunol Rev. 1988;101:133–148. doi: 10.1111/j.1600-065x.1988.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 28.Stall A M, Wells S M, Lam K-P. Semin Immunol. 1996;8:45–59. doi: 10.1006/smim.1996.0007. [DOI] [PubMed] [Google Scholar]
- 29.Pillai S. Immunity. 1999;10:493–502. doi: 10.1016/s1074-7613(00)80049-7. [DOI] [PubMed] [Google Scholar]
- 30.Andreu-Sanchez J L, Faro J, Alonso J M, Paige C J, Martinez A, C, Marcos M A. Eur J Immunol. 1990;20:1767–1773. doi: 10.1002/eji.1830200822. [DOI] [PubMed] [Google Scholar]
- 31.Philpott K L, Viney J L, Kay G, Rastan S, Gardiner E M, Chae S, Hayday A C, Owen M J. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 32.Rathmell J C, Townsend S E, Xu J C, Flavell R A, Goodnow C C. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 33.Vignaux F, Golstein P. Eur J Immunol. 1994;24:923–927. doi: 10.1002/eji.1830240421. [DOI] [PubMed] [Google Scholar]
- 34.Lortan J E, Roobottom C A, Oldfield S, MacLennan I C. Eur J Immunol. 1987;17:1311–1316. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- 35.Allman D M, Ferguson S E, Lentz V M, Cancro M P. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 36.Chan E Y, MacLennan I C. Eur J Immunol. 1993;23:357–363. doi: 10.1002/eji.1830230209. [DOI] [PubMed] [Google Scholar]
- 37.Foerster I, Rajewsky K. Proc Natl Acad Sci USA. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook M C, Basten A, Fazekas de St. Groth B. J Exp Med. 1997;186:631–643. doi: 10.1084/jem.186.5.631. .B. F. D. S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cyster J G, Goodnow C C. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 40.Hartley S S, Crosbie J, Brink R, Kantor A B, Basten A, Goodnow C C. Nature (London) 1991;353:765–768. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 41.Hartley S B, Cooke M P, Fulcher D A, Harris A W, Cory S, Basten A, Goodnow C C. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 42.Melamed D, Benschop R J, Cambier J C, Nemazee D. Cell. 1998;92:173–182. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- 43.Monroe J G. J Immunol. 1996;156:2657–2660. [PubMed] [Google Scholar]
- 44.Merino R, Ding L, Veis D J, Korsmeyer S J, Nunez G. EMBO J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sater R A, Sandel P C, Monroe J G. Int Immunol. 1998;10:1673–1682. doi: 10.1093/intimm/10.11.1673. [DOI] [PubMed] [Google Scholar]