Abstract
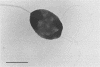
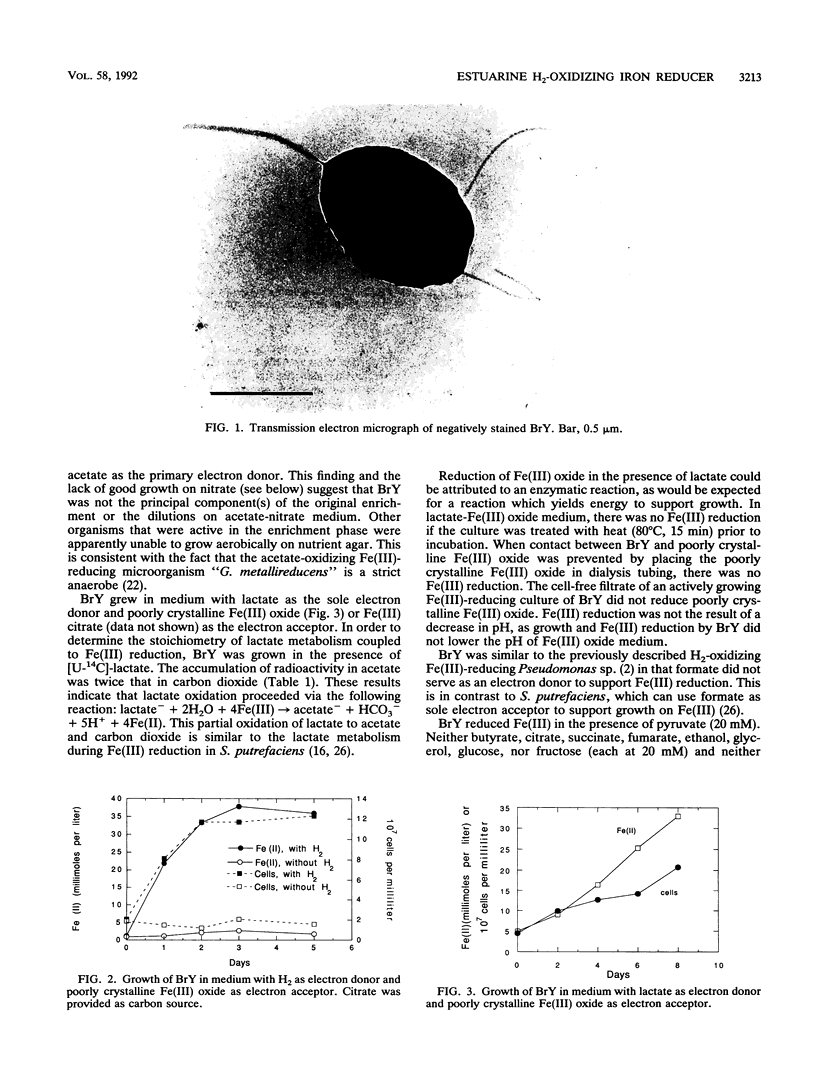
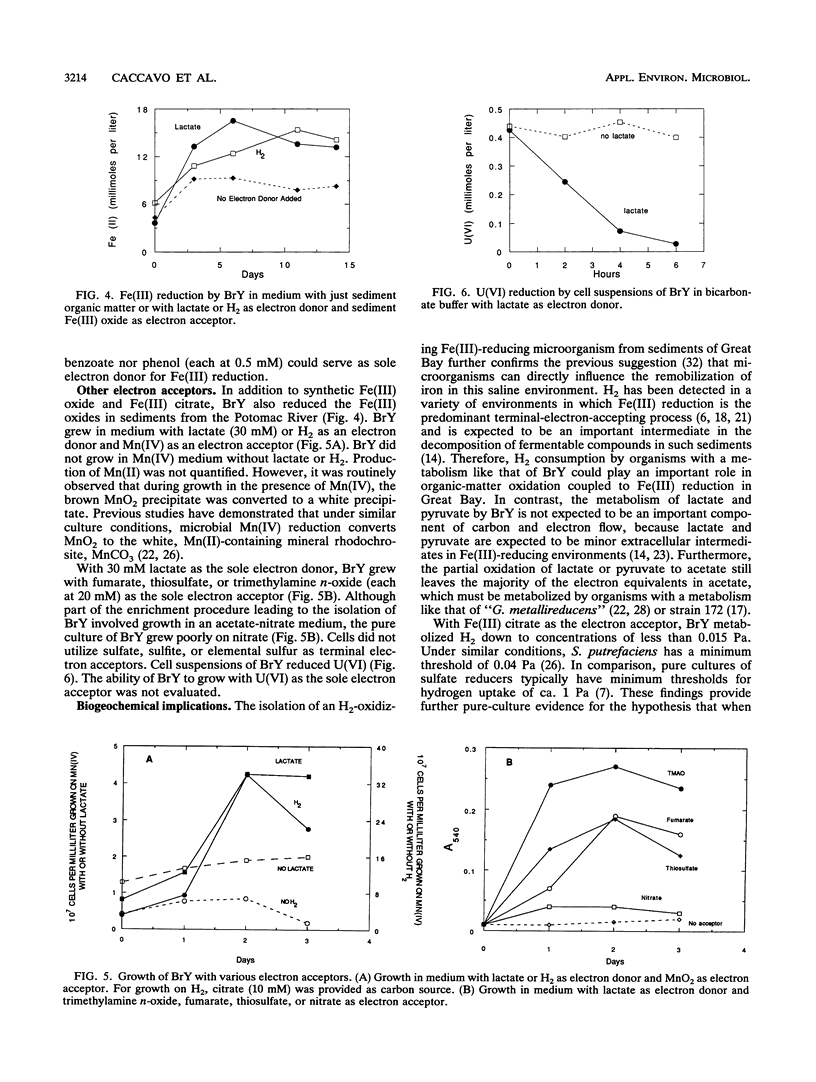
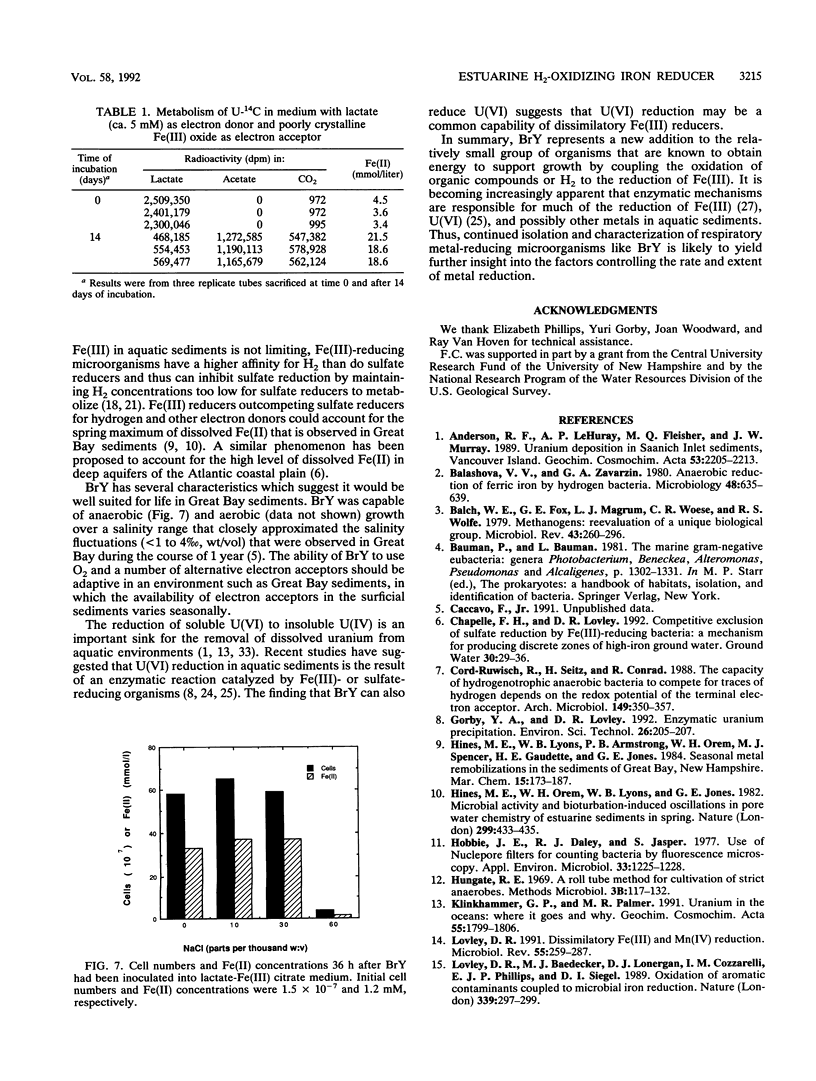
A dissimilatory Fe(III)- and Mn(IV)-reducing bacterium was isolated from bottom sediments of the Great Bay estuary, New Hampshire. The isolate was a facultatively anaerobic gram-negative rod which did not appear to fit into any previously described genus. It was temporarily designated strain BrY. BrY grew anaerobically in a defined medium with hydrogen or lactate as the electron donor and Fe(III) as the electron acceptor. BrY required citrate, fumarate, or malate as a carbon source for growth on H2 and Fe(III). With Fe(III) as the sole electron acceptor, BrY metabolized hydrogen to a minimum threshold at least 60-fold lower than the threshold reported for pure cultures of sulfate reducers. This finding supports the hypothesis that when Fe(III) is available, Fe(III) reducers can outcompete sulfate reducers for electron donors. Lactate was incompletely oxidized to acetate and carbon dioxide with Fe(III) as the electron acceptor. Lactate oxidation was also coupled to the reduction of Mn(IV), U(VI), fumarate, thiosulfate, or trimethylamine n-oxide under anaerobic conditions. BrY provides a model for how enzymatic metal reduction by respiratory metal-reducing microorganisms has the potential to contribute to the mobilization of iron and trace metals and to the immobilization of uranium in sediments of Great Bay Estuary.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991 Jun;55(2):259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Lonergan D. J. Anaerobic Oxidation of Toluene, Phenol, and p-Cresol by the Dissimilatory Iron-Reducing Organism, GS-15. Appl Environ Microbiol. 1990 Jun;56(6):1858–1864. doi: 10.1128/aem.56.6.1858-1864.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J., Caccavo F., Jr Acetate oxidation by dissimilatory Fe(III) reducers. Appl Environ Microbiol. 1992 Sep;58(9):3205–3208. doi: 10.1128/aem.58.9.3205-3208.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol. 1987 Nov;53(11):2636–2641. doi: 10.1128/aem.53.11.2636-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J., Lonergan D. J. Hydrogen and Formate Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese by Alteromonas putrefaciens. Appl Environ Microbiol. 1989 Mar;55(3):700–706. doi: 10.1128/aem.55.3.700-706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988 Jun;54(6):1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Reduction of uranium by Desulfovibrio desulfuricans. Appl Environ Microbiol. 1992 Mar;58(3):850–856. doi: 10.1128/aem.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Requirement for a Microbial Consortium To Completely Oxidize Glucose in Fe(III)-Reducing Sediments. Appl Environ Microbiol. 1989 Dec;55(12):3234–3236. doi: 10.1128/aem.55.12.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostle A. G., Holt J. G. Nile blue A as a fluorescent stain for poly-beta-hydroxybutyrate. Appl Environ Microbiol. 1982 Jul;44(1):238–241. doi: 10.1128/aem.44.1.238-241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugel J. B., Hines M. E., Jones G. E. Microbial iron reduction by enrichment cultures isolated from estuarine sediments. Appl Environ Microbiol. 1986 Nov;52(5):1167–1172. doi: 10.1128/aem.52.5.1167-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]