Abstract
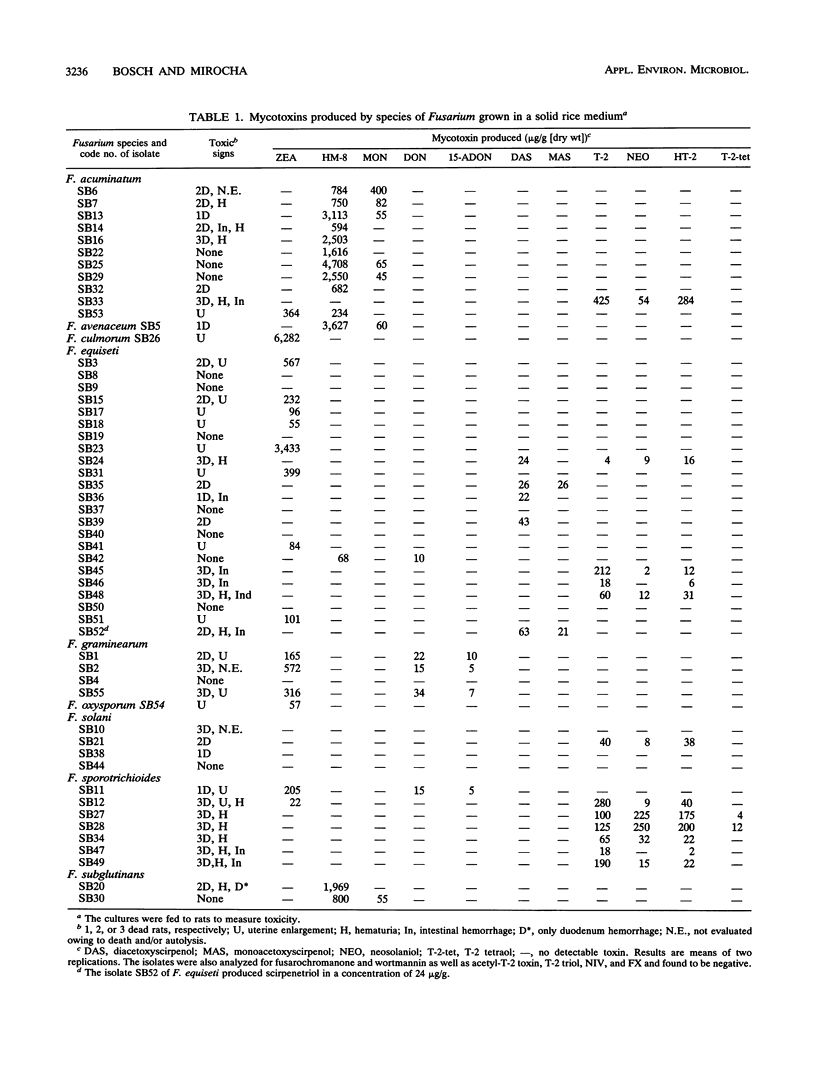
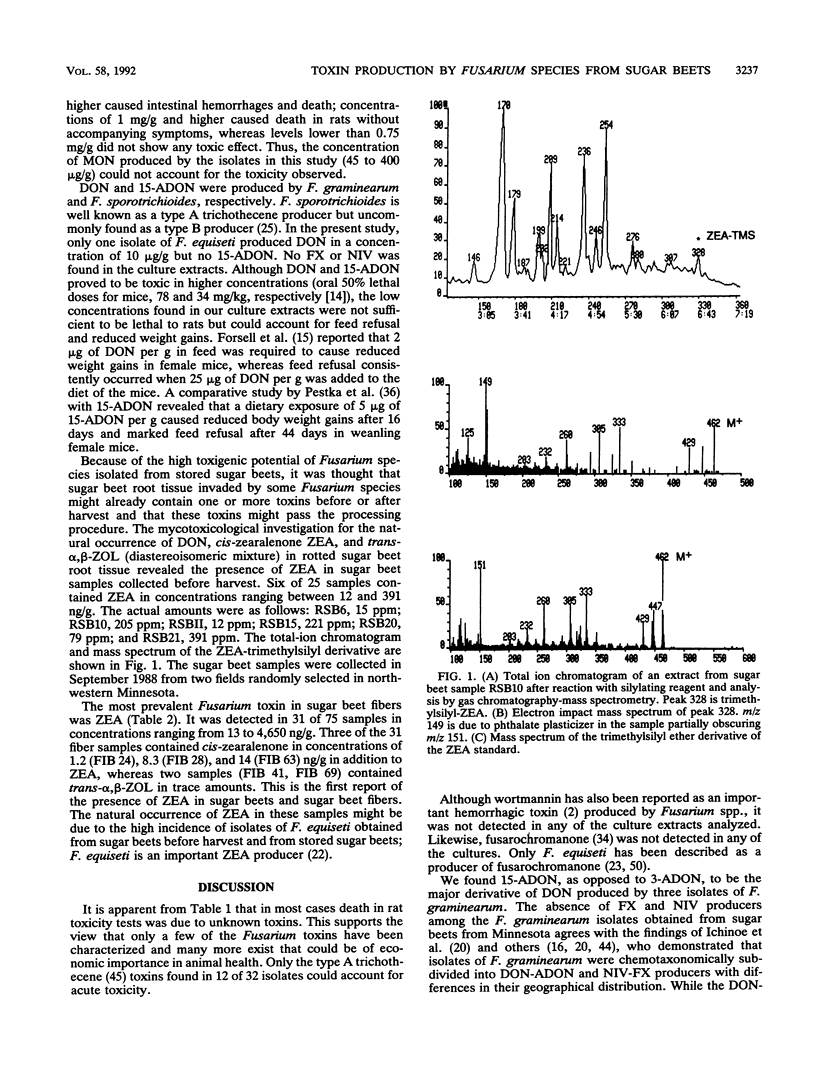
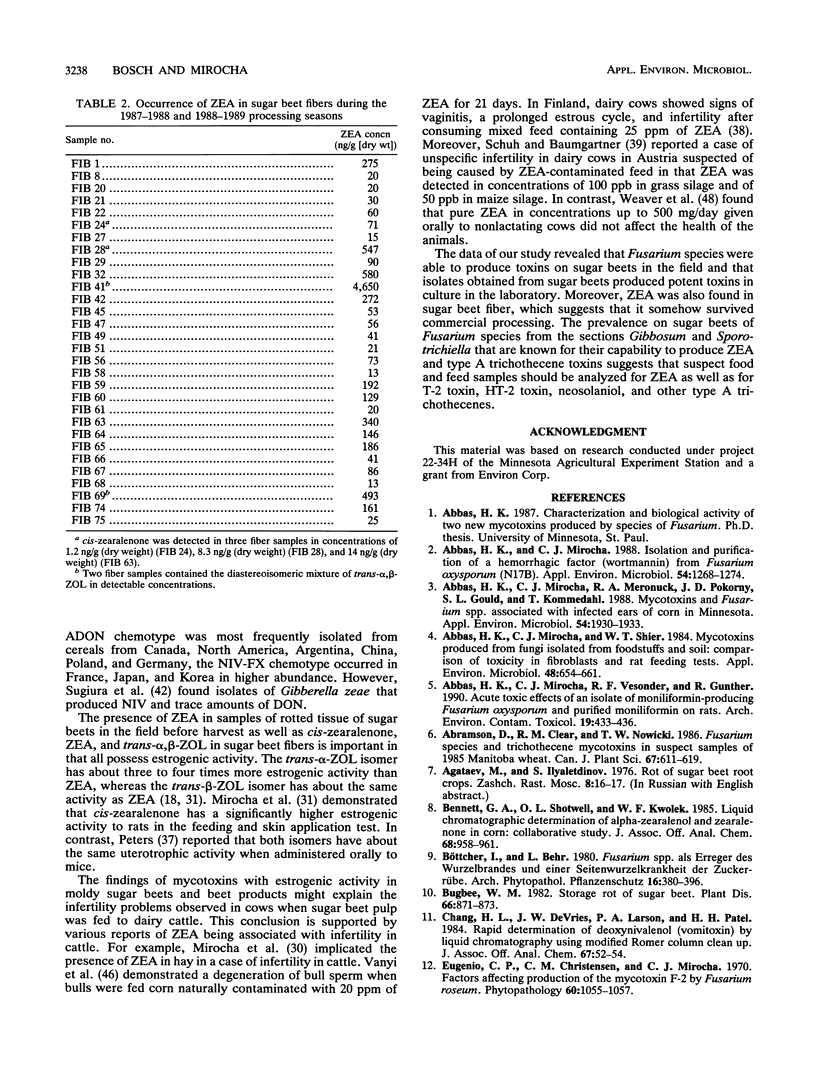
Fifty-five Fusarium isolates belonging to nine species were collected from fungus-invaded tissue of stored sugar beets and identified as F. acuminatum (11 isolates), F. avenaceum (1 isolate), F. culmorum (1 isolate), F. equiseti (23 isolates), F. graminearum (4 isolates), F. oxysporum (1 isolate), F. solani (4 isolates), F. sporotrichioides (7 isolates), and F. subglutinans (2 isolates). All isolates were cultured on autoclaved rice grains and assayed for toxicity by feeding weanling female rats the ground-rice cultures of the isolates in a 50% mixture with a regular diet for 5 days. Fifty-eight percent of the isolates were acutely toxic to rats, 26% caused hematuria, 18% caused hemorrhages, and 29% caused uterine enlargement. In most cases, toxicity could not be accounted for by the known toxins found. The following mycotoxins were found in extracts of the rice cultures: zearalenone (22 to 6,282 micrograms/g), chlamydosporol (HM-8) (68 to 4,708 micrograms/g), moniliformin (45 to 400 micrograms/g), deoxynivalenol (10 to 34 micrograms/g), 15-acetyldeoxynivalenol (5 to 10 micrograms/g), diacetoxyscirpenol (22 to 63 micrograms/g), monoacetoxyscirpenol (21 to 26 micrograms/g), scirpenetriol (24 micrograms/g), T-2 toxin (4 to 425 micrograms/g), HT-2 toxin (2 to 284 micrograms/g), neosolaniol (2 to 250 micrograms/g), and T-2 tetraol (4 to 12 micrograms/g). F. equiseti was the predominant species found on visibly molded beets in the field. Six of 25 moldy sugar beet root samples collected in the field contained zearalenone in concentrations ranging between 12 and 391 ng/g, whereas 10 samples from commercial stockpiles were negative for zearalenone.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas H. K., Mirocha C. J. Isolation and purification of a hemorrhagic factor (wortmannin) from Fusarium oxysporum (N17B). Appl Environ Microbiol. 1988 May;54(5):1268–1274. doi: 10.1128/aem.54.5.1268-1274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas H. K., Mirocha C. J., Meronuck R. A., Pokorny J. D., Gould S. L., Kommedahl T. Mycotoxins and Fusarium spp. associated with infected ears of corn in Minnesota. Appl Environ Microbiol. 1988 Aug;54(8):1930–1933. doi: 10.1128/aem.54.8.1930-1933.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas H. K., Mirocha C. J., Shier W. T. Mycotoxins produced from fungi isolated from foodstuffs and soil: comparison of toxicity in fibroblasts and rat feeding tests. Appl Environ Microbiol. 1984 Sep;48(3):654–661. doi: 10.1128/aem.48.3.654-661.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas H. K., Mirocha C. J., Vesonder R. F., Gunther R. Acute toxic effects of an isolate of moniliformin-producing Fusarium oxysporum and purified moniliformin on rats. Arch Environ Contam Toxicol. 1990 May-Jun;19(3):433–436. doi: 10.1007/BF01054989. [DOI] [PubMed] [Google Scholar]
- Bennett G. A., Shotwell O. L., Kwolek W. F. Liquid chromatographic determination of alpha-zearalenol and zearalenone in corn: collaborative study. J Assoc Off Anal Chem. 1985 Sep-Oct;68(5):958–961. [PubMed] [Google Scholar]
- Chang H. L., DeVries J. W., Larson P. A., Patel H. H. Rapid determination of deoxynivalenol (vomitoxin) by liquid chromatography using modified Romer column cleanup. J Assoc Off Anal Chem. 1984 Jan-Feb;67(1):52–54. [PubMed] [Google Scholar]
- Eugenio C. P., Christensen C. M., Mirocha C. J. Factors affecting production of the mycotoxin F-2 by Fusarium roseum. Phytopathology. 1970 Jul;(7):1055–1057. doi: 10.1094/phyto-60-1055. [DOI] [PubMed] [Google Scholar]
- Forsell J. H., Jensen R., Tai J. H., Witt M., Lin W. S., Pestka J. J. Comparison of acute toxicities of deoxynivalenol (vomitoxin) and 15-acetyldeoxynivalenol in the B6C3F1 mouse. Food Chem Toxicol. 1987 Feb;25(2):155–162. doi: 10.1016/0278-6915(87)90149-9. [DOI] [PubMed] [Google Scholar]
- Forsell J. H., Witt M. F., Tai J. H., Jensen R., Pestka J. J. Effects of 8-week exposure of the B6C3F1 mouse to dietary deoxynivalenol (vomitoxin) and zearalenone. Food Chem Toxicol. 1986 Mar;24(3):213–219. doi: 10.1016/0278-6915(86)90231-0. [DOI] [PubMed] [Google Scholar]
- Greenhalgh R., Neish G. A., Miller J. D. Deoxynivalenol, acetyl deoxynivalenol, and zearalenone formation by Canadian isolates of Fusarium graminearum on solid substrates. Appl Environ Microbiol. 1983 Sep;46(3):625–629. doi: 10.1128/aem.46.3.625-629.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler W. M., Mirocha C. J., Pathre S. V., Behrens J. C. Identification of the naturally occurring isomer of zearalenol produced by Fusarium roseum 'Gibbosum' in rice culture. Appl Environ Microbiol. 1979 May;37(5):849–853. doi: 10.1128/aem.37.5.849-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinoe M., Kurata H., Sugiura Y., Ueno Y. Chemotaxonomy of Gibberella zeae with special reference to production of trichothecenes and zearalenone. Appl Environ Microbiol. 1983 Dec;46(6):1364–1369. doi: 10.1128/aem.46.6.1364-1369.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper-Goodman T., Scott P. M., Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul Toxicol Pharmacol. 1987 Sep;7(3):253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- Lee Y. W., Mirocha C. J., Shroeder D. J., Walser M. M. TDP-1, a toxic component causing tibial dyschondroplasia in broiler chickens, and trichothecenes from Fusarium roseum 'Graminearum'. Appl Environ Microbiol. 1985 Jul;50(1):102–107. doi: 10.1128/aem.50.1.102-107.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirocha C. J., Abbas H. K., Windels C. E., Xie W. Variation in Deoxynivalenol, 15-Acetyldeoxynivalenol, 3-Acetyldeoxynivalenol, and Zearalenone Production by Fusarium graminearum Isolates. Appl Environ Microbiol. 1989 May;55(5):1315–1316. doi: 10.1128/aem.55.5.1315-1316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirocha C. J., Harrison J., Nichols A. A., McClintock M. Detection of a fungal estrogen (F-2) in hay associated with infertility in dairy cattle. Appl Microbiol. 1968 May;16(5):797–798. doi: 10.1128/am.16.5.797-798.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirocha C. J., Pathre S. V., Behrens J., Schauerhamer B. Uterotropic activity of cis and trans isomers of zearalenone and zearalenol. Appl Environ Microbiol. 1978 May;35(5):986–987. doi: 10.1128/aem.35.5.986-987.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka J. J., Lin W. S., Forsell J. H. Decreased feed consumption and body-weight gain in the B6C3F1 mouse after dietary exposure to 15-acetyldeoxynivalenol. Food Chem Toxicol. 1986 Dec;24(12):1309–1313. doi: 10.1016/0278-6915(86)90063-3. [DOI] [PubMed] [Google Scholar]
- Peters C. A. Photochemistry of zearalenone and its derivatives. J Med Chem. 1972 Aug;15(8):867–868. doi: 10.1021/jm00278a028. [DOI] [PubMed] [Google Scholar]
- Scott P. M., Lawrence G. A. Liquid chromatographic determination and stability of the Fusarium mycotoxin moniliformin in cereal grains. J Assoc Off Anal Chem. 1987 Sep-Oct;70(5):850–853. [PubMed] [Google Scholar]
- Sugiura Y., Watanabe Y., Tanaka T., Yamamoto S., Ueno Y. Occurrence of Gibberella zeae strains that produce both nivalenol and deoxynivalenol. Appl Environ Microbiol. 1990 Oct;56(10):3047–3051. doi: 10.1128/aem.56.10.3047-3051.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Hasegawa A., Matsuki Y., Ueno Y. A survey of the occurrence of nivalenol, deoxynivalenol and zearalenone in food stuffs and health foods in Japan. Food Addit Contam. 1985 Oct-Dec;2(4):259–265. doi: 10.1080/02652038509373554. [DOI] [PubMed] [Google Scholar]
- Weaver G. A., Kurtz H. J., Behrens J. C., Robison T. S., Seguin B. E., Bates F. Y., Mirocha C. J. Effect of zearalenone on dairy cows. Am J Vet Res. 1986 Aug;47(8):1826–1828. [PubMed] [Google Scholar]


