Abstract
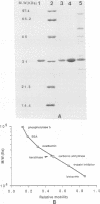
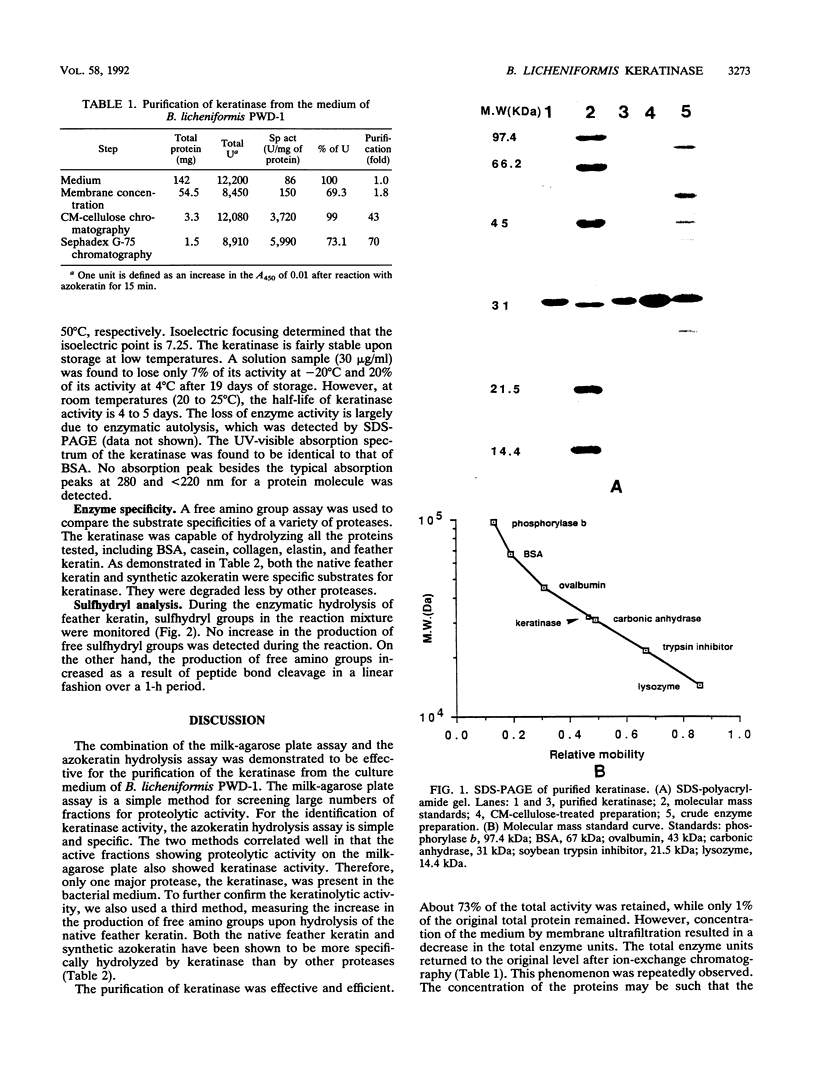
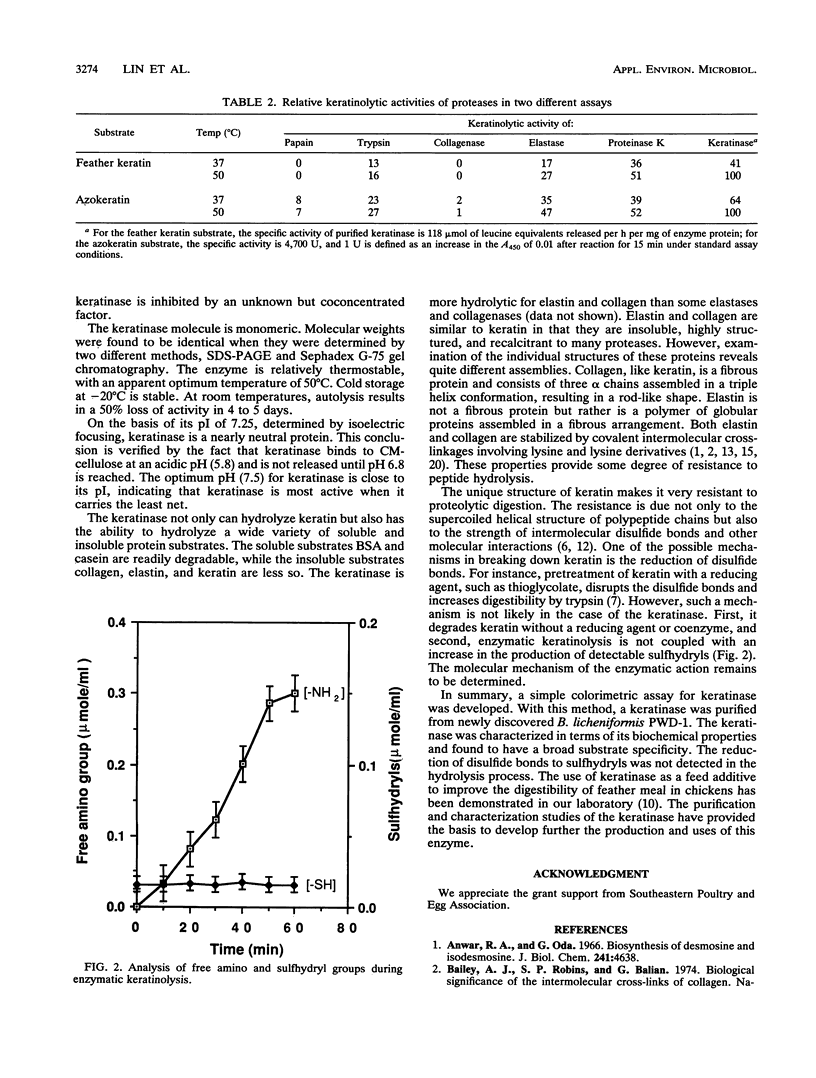
A keratinase was isolated from the culture medium of feather-degrading Bacillus licheniformis PWD-1 by use of an assay of the hydrolysis of azokeratin. Membrane ultrafiltration and carboxymethyl cellulose ion-exchange and Sephadex G-75 gel chromatographies were used to purify the enzyme. The specific activity of the purified keratinase relative to that in the original medium was approximately 70-fold. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and Sephadex G-75 chromatography indicated that the purified keratinase is monomeric and has a molecular mass of 33 kDa. The optimum pH and the pI were determined to be 7.5 and 7.25, respectively. Under standard assay conditions, the apparent temperature optimum was 50°C. The enzyme is stable when stored at −20°C. The purified keratinase hydrolyzes a broad range of substrates and displays higher proteolytic activity than most proteases. In practical applications, keratinase is a useful enzyme for promoting the hydrolysis of feather keratin and improving the digestibility of feather meal.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar R. A., Oda G. The biosynthesis of desmosine and isodesmosine. J Biol Chem. 1966 Oct 25;241(20):4638–4641. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Harrap B. S., Woods E. F. Soluble derivatives of feather keratin. 2. Molecular weight and conformation. Biochem J. 1964 Jul;92(1):19–26. doi: 10.1042/bj0920019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehta P. D., Patrick B. A. Detection of oligoclonal bands in unconcentrated CSF: isoelectric focusing and silver staining. Neurology. 1983 Oct;33(10):1365–1368. doi: 10.1212/wnl.33.10.1365. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Martin G. R., Mecca C. E., Piez K. A. The biosynthesis of elastin cross-links. The effect of copper deficiency and a lathyrogen. J Biol Chem. 1965 Sep;240(9):3623–3627. [PubMed] [Google Scholar]
- Parry D. A., Crewther W. G., Fraser R. D., MacRae T. P. Structure of alpha-keratin: structural implication of the amino acid sequences of the type I and type II chain segments. J Mol Biol. 1977 Jun 25;113(2):449–454. doi: 10.1016/0022-2836(77)90153-x. [DOI] [PubMed] [Google Scholar]
- Partridge S. M., Elsden D. F., Thomas J., Dorfman A., Telser A., Ho P. L. Incorporation of labelled lysine into the desmosine cross-bridges in elastin. Nature. 1966 Jan 22;209(5021):399–400. doi: 10.1038/209399b0. [DOI] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Sandholm M., Smith R. R., Shih J. C., Scott M. L. Determination of antitrypsin activity on agar plates: relationship between antitrypsin and biological value of soybean for trout. J Nutr. 1976 Jun;106(6):761–766. doi: 10.1093/jn/106.6.761. [DOI] [PubMed] [Google Scholar]
- Williams C. M., Richter C. S., Mackenzie J. M., Shih J. C. Isolation, identification, and characterization of a feather-degrading bacterium. Appl Environ Microbiol. 1990 Jun;56(6):1509–1515. doi: 10.1128/aem.56.6.1509-1515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]