Abstract
The enzyme 3-hydroxybutyryl-coenzyme A (CoA) dehydrogenase has been purified 45-fold to apparent homogeneity from the solvent-producing anaerobe Clostridium beijerinckii NRRL B593. The identities of 34 of the N-terminal 35 amino acid residues have been determined. The enzyme exhibited a native M(r) of 213,000 and a subunit M(r) of 30,800. It is specific for the (S)-enantiomer of 3-hydroxybutyryl-CoA. Michaelis constants for NADH and acetoacetyl-CoA were 8.6 and 14 microM, respectively. The maximum velocity of the enzyme was 540 mumol min-1 mg-1 for the reduction of acetoacetyl-CoA with NADH. The enzyme could use either NAD(H) or NADP(H) as a cosubstrate; however, kcat/Km for the NADH-linked reaction was much higher than the apparent value for the NADPH-linked reaction. Also, NAD(H)-linked activity was less sensitive to changes in pH than NADP(H)-linked activity was. In the presence of 9.5 microM NADH, the enzyme was inhibited by acetoacetyl-CoA at concentrations as low as 20 microM, but the inhibition was relieved as the concentration of NADH was increased, suggesting a possible mechanism for modulating the energy efficiency during growth.
Full text
PDF

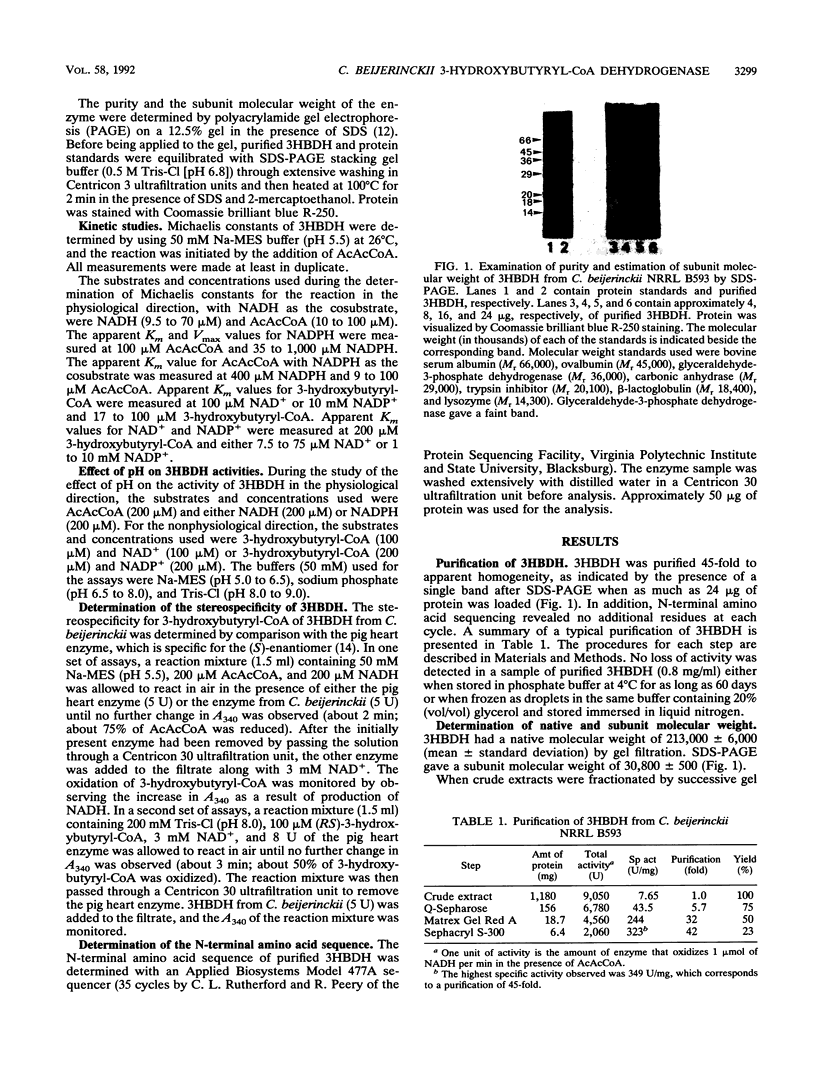
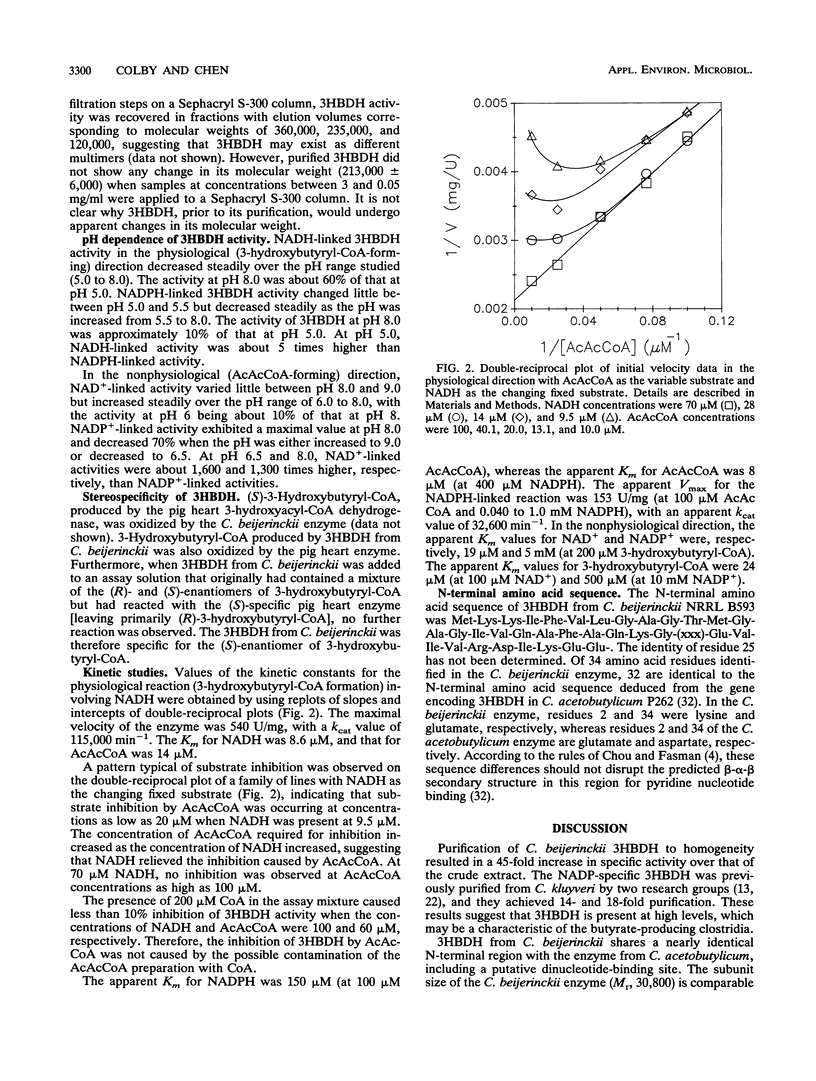


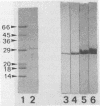
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birktoft J. J., Holden H. M., Hamlin R., Xuong N. H., Banaszak L. J. Structure of L-3-hydroxyacyl-coenzyme A dehydrogenase: preliminary chain tracing at 2.8-A resolution. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8262–8266. doi: 10.1073/pnas.84.23.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles L. K., Ellefson W. L. Effects of butanol on Clostridium acetobutylicum. Appl Environ Microbiol. 1985 Nov;50(5):1165–1170. doi: 10.1128/aem.50.5.1165-1170.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Fukui T., Ito M., Saito T., Tomita K. Purification and characterization of NADP-linked acetoacetyl-CoA reductase from Zoogloea ramigera I-16-M. Biochim Biophys Acta. 1987 Feb 23;917(3):365–371. [PubMed] [Google Scholar]
- George H. A., Johnson J. L., Moore W. E., Holdeman L. V., Chen J. S. Acetone, Isopropanol, and Butanol Production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl Environ Microbiol. 1983 Mar;45(3):1160–1163. doi: 10.1128/aem.45.3.1160-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T. Individual peroxisomal beta-oxidation enzymes. Ann N Y Acad Sci. 1982;386:5–12. doi: 10.1111/j.1749-6632.1982.tb21403.x. [DOI] [PubMed] [Google Scholar]
- Huang L., Gibbins L. N., Forsberg C. W. Transmembrane pH gradient and membrane potential in Clostridium acetobutylicum during growth under acetogenic and solventogenic conditions. Appl Environ Microbiol. 1985 Oct;50(4):1043–1047. doi: 10.1128/aem.50.4.1043-1047.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madan V. K., Hillmer P., Gottschalk G. Purification and properties of NADP-dependent L(+)-3-hydroxybutyryl-CoA dehydrogenase from Clostridium kluyveri. Eur J Biochem. 1973 Jan 3;32(1):51–56. doi: 10.1111/j.1432-1033.1973.tb02577.x. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Bradshaw R. A. L-3-hydroxyacyl coenzyme A dehydrogenase from pig heart muscle. I. Purification and properties. J Biol Chem. 1973 May 10;248(9):3052–3059. [PubMed] [Google Scholar]
- Noyes B. E., Bradshaw R. A. L-3-hydroxyacyl coenzyme A dehydrogenase from pig heart muscle. II. Subunit structure. J Biol Chem. 1973 May 10;248(9):3061–3066. [PubMed] [Google Scholar]
- Osumi T., Hashimoto T. Purification and properties of mitochondrial and peroxisomal 3-hydroxyacyl-CoA dehydrogenase from rat liver. Arch Biochem Biophys. 1980 Aug;203(1):372–383. doi: 10.1016/0003-9861(80)90189-7. [DOI] [PubMed] [Google Scholar]
- Prasad M. R., Cook L., Vieth R., Cinti D. L. Rat hepatic microsomal acetoacetyl-CoA reductase. A beta-ketoacyl-CoA reductase distinct from the long chain beta-ketoacyl-CoA reductase component of the microsomal fatty acid chain elongation system. J Biol Chem. 1984 Jun 25;259(12):7460–7467. [PubMed] [Google Scholar]
- Ritchie G. A., Senior P. J., Dawes E. A. The purification and characterization of acetoacetyl-coenzyme A reductase from Azotobacter beijerinckii. Biochem J. 1971 Jan;121(2):309–316. doi: 10.1042/bj1210309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J. R. Crystalline beta-hydroxybutyryl dehydrogenase from pig heart. Biochim Biophys Acta. 1957 Nov;26(2):448–449. doi: 10.1016/0006-3002(57)90040-9. [DOI] [PubMed] [Google Scholar]
- STERN J. R. Optical properties of aceto-acetyl-S-coenzyme A and its metal chelates. J Biol Chem. 1956 Jul;221(1):33–44. [PubMed] [Google Scholar]
- Shuto H., Fukui T., Saito T., Shirakura Y., Tomita K. An NAD-linked acetoacetyl-CoA reductase from Zoogloea ramigera I-16-M. Eur J Biochem. 1981 Aug;118(1):53–59. doi: 10.1111/j.1432-1033.1981.tb05485.x. [DOI] [PubMed] [Google Scholar]
- Sliwkowski M. X., Hartmanis M. G. Simultaneous single-step purification of thiolase and NADP-dependent 3-hydroxybutyryl-CoA dehydrogenase from Clostridium kluyveri. Anal Biochem. 1984 Sep;141(2):344–347. doi: 10.1016/0003-2697(84)90052-6. [DOI] [PubMed] [Google Scholar]
- Terracciano J. S., Kashket E. R. Intracellular Conditions Required for Initiation of Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):86–91. doi: 10.1128/aem.52.1.86-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. K., Chen J. S. Purification and properties of an acetoacetyl coenzyme A-reacting phosphotransbutyrylase from Clostridium beijerinckii ("Clostridium butylicum") NRRL B593. Appl Environ Microbiol. 1990 Mar;56(3):607–613. doi: 10.1128/aem.56.3.607-613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIL S. J., GREEN D. E., MII S., MAHLER H. R. Studies on the fatty acid oxidizing system of animal tissues. VI. beta-Hydroxyacyl coenzyme A dehydrogenase. J Biol Chem. 1954 Apr;207(2):631–638. [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Coenzyme A transferase from Clostridium acetobutylicum ATCC 824 and its role in the uptake of acids. Appl Environ Microbiol. 1989 Feb;55(2):323–329. doi: 10.1128/aem.55.2.323-329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenborn D. P., Rudolph F. B., Papoutsakis E. T. Thiolase from Clostridium acetobutylicum ATCC 824 and Its Role in the Synthesis of Acids and Solvents. Appl Environ Microbiol. 1988 Nov;54(11):2717–2722. doi: 10.1128/aem.54.11.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Run-Tao, Zhu Chang-Xi, Golemboski Christine, Chen Jiann-Shin. Expression of Solvent-Forming Enzymes and Onset of Solvent Production in Batch Cultures of Clostridium beijerinckii ("Clostridium butylicum"). Appl Environ Microbiol. 1988 Mar;54(3):642–648. doi: 10.1128/aem.54.3.642-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. Y., Schulz H., Elzinga M., Yang S. Y. Nucleotide sequence of the promoter and fadB gene of the fadBA operon and primary structure of the multifunctional fatty acid oxidation protein from Escherichia coli. Biochemistry. 1991 Jul 9;30(27):6788–6795. doi: 10.1021/bi00241a023. [DOI] [PubMed] [Google Scholar]
- Youngleson J. S., Jones D. T., Woods D. R. Homology between hydroxybutyryl and hydroxyacyl coenzyme A dehydrogenase enzymes from Clostridium acetobutylicum fermentation and vertebrate fatty acid beta-oxidation pathways. J Bacteriol. 1989 Dec;171(12):6800–6807. doi: 10.1128/jb.171.12.6800-6807.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]