Abstract
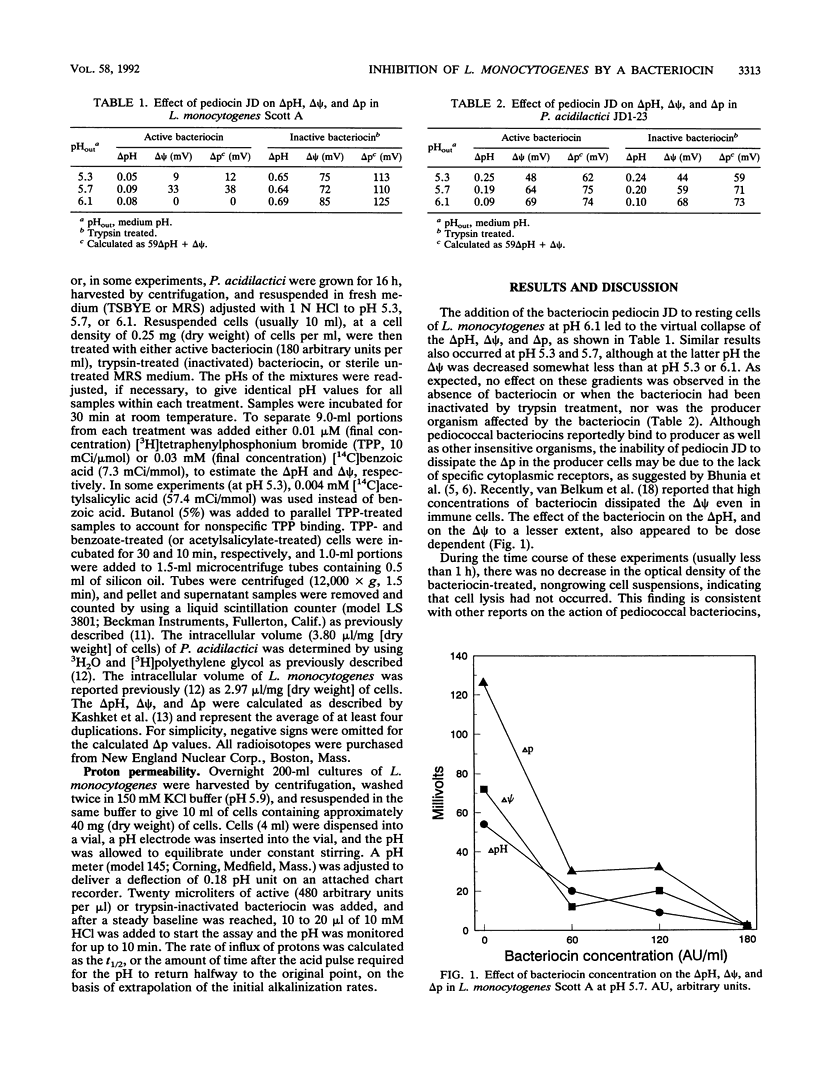
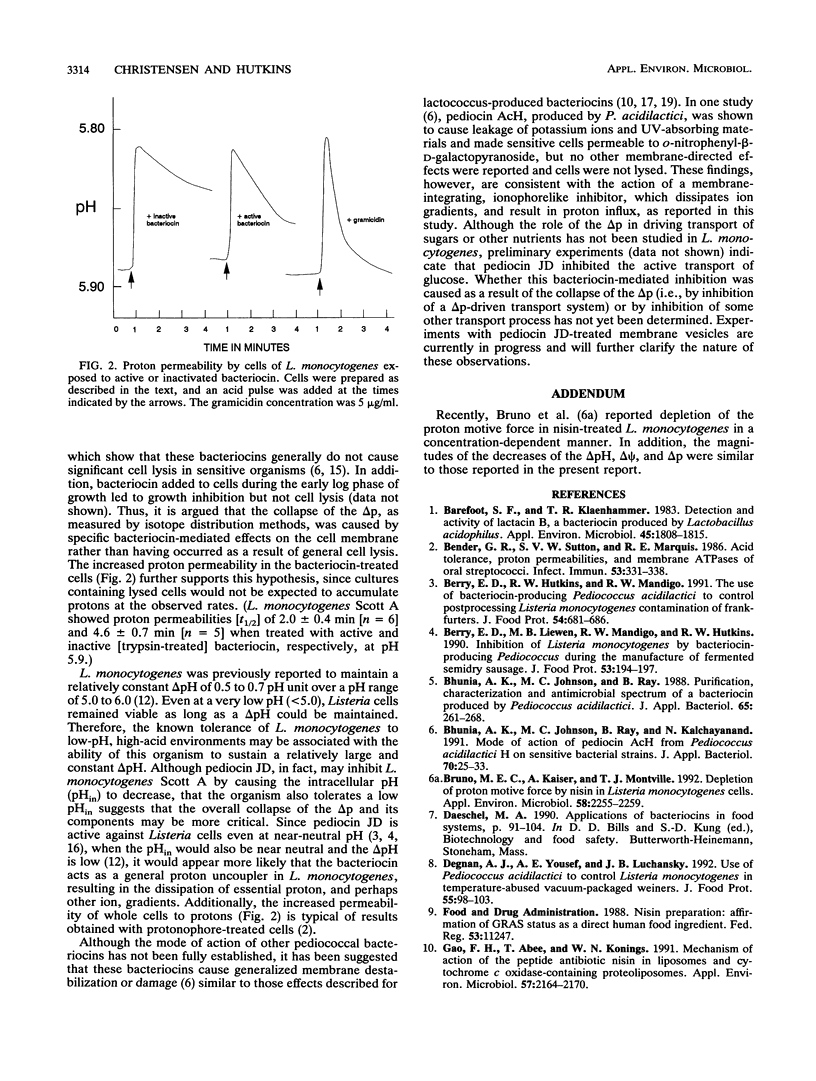
The effect of pediocin JD, a bacteriocin produced by Pediococcus acidilactici JD1-23, on the proton motive force and proton permeability of resting whole cells of Listeria monocytogenes Scott A was determined. Control cells, treated with trypsin-inactivated bacteriocin at a pH of 5.3 to 6.1, maintained a pH gradient and a membrane potential of approximately 0.65 pH unit and 75 mV, respectively. However, these gradients were rapidly dissipated in cells after exposure to pediocin JD, even though no cell lysis had occurred. The pH gradient and membrane potential of the producer cells were also unaffected by the bacteriocin. Whole cells treated with bacteriocin were twice as permeable to protons as control cells were. The results suggest that the inhibitory action of pediocin JD against L. monocytogenes is directed at the cytoplasmic membrane and that inhibition of L. monocytogenes may be caused by the collapse of one or both of the individual components of the proton motive force.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barefoot S. F., Klaenhammer T. R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol. 1983 Jun;45(6):1808–1815. doi: 10.1128/aem.45.6.1808-1815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender G. R., Sutton S. V., Marquis R. E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986 Aug;53(2):331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunia A. K., Johnson M. C., Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988 Oct;65(4):261–268. doi: 10.1111/j.1365-2672.1988.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Bruno M. E., Kaiser A., Montville T. J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992 Jul;58(7):2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutkins R. W., Ponne C. Lactose Uptake Driven by Galactose Efflux in Streptococcus thermophilus: Evidence for a Galactose-Lactose Antiporter. Appl Environ Microbiol. 1991 Apr;57(4):941–944. doi: 10.1128/aem.57.4.941-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke A., Montville T. J. Bacteriocin-mediated inhibition of Clostridium botulinum spores by lactic acid bacteria at refrigeration and abuse temperatures. Appl Environ Microbiol. 1991 Dec;57(12):3423–3428. doi: 10.1128/aem.57.12.3423-3428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Vedamuthu E. R., Kunka B. S., Vandenbergh P. A. Inhibition of Listeria monocytogenes by using bacteriocin PA-1 produced by Pediococcus acidilactici PAC 1.0. Appl Environ Microbiol. 1988 Oct;54(10):2349–2353. doi: 10.1128/aem.54.10.2349-2353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel J. K., Ceglowski P., Dobrazański W. T. Mechanism of action of lactostrepcin 5, a bacteriocin produced by Streptococcus cremoris 202. Appl Environ Microbiol. 1985 Apr;49(4):969–974. doi: 10.1128/aem.49.4.969-974.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Kok J., Venema G., Holo H., Nes I. F., Konings W. N., Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991 Dec;173(24):7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



