Abstract
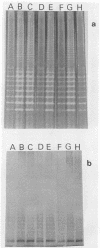
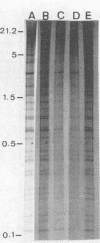
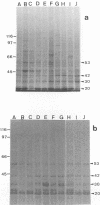
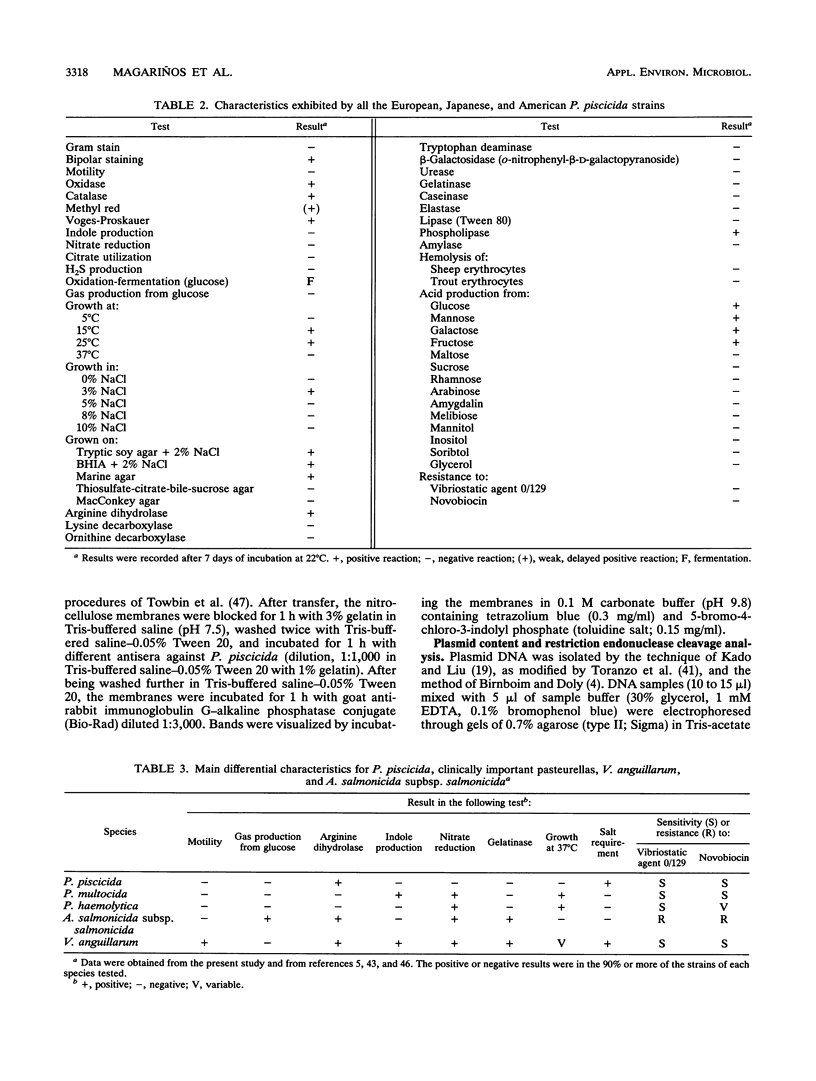
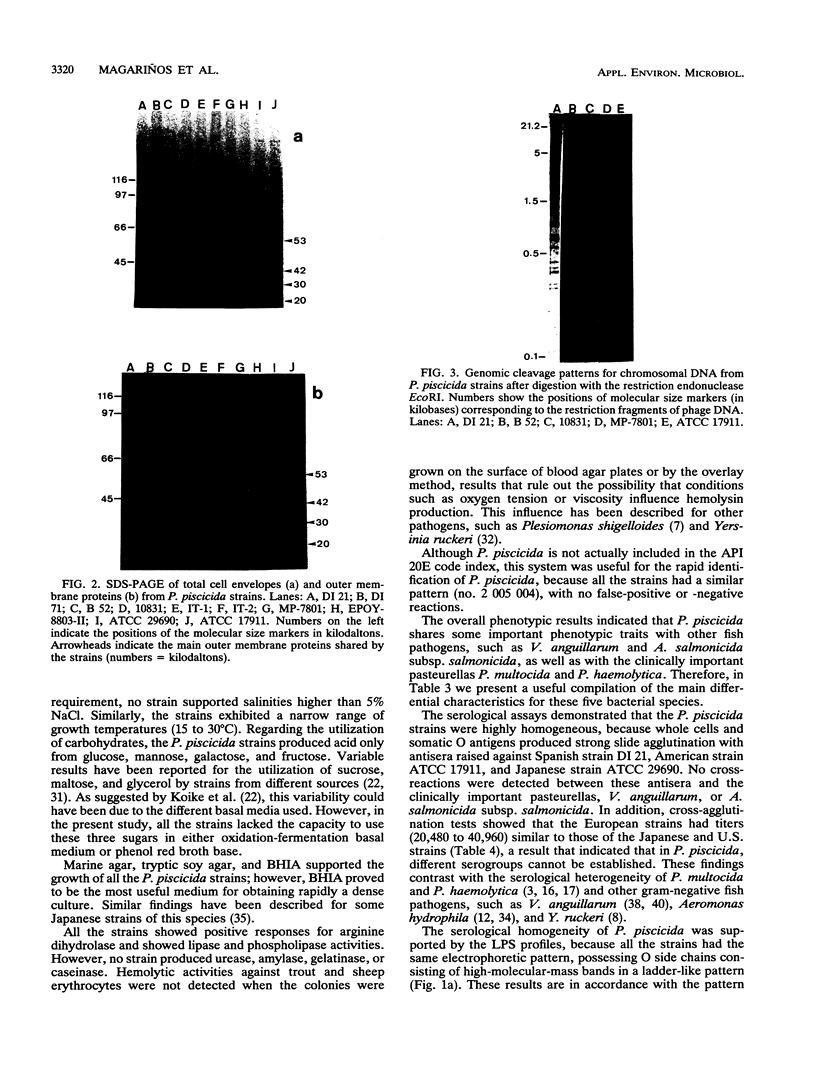
We compared Pasteurella piscicida strains isolated from different fish species in several European countries with strains isolated in Japan and the United States. The taxonomic analysis revealed that, regardless of the geographic origin and source of isolation, all the strains exhibited the same biochemical and physiological characteristics. Serological assays with different rabbit antisera demonstrated a high level of antigenic similarity among strains, with cross-agglutination titers of 20,480 to 40,960. This serological homogeneity was supported by the lipopolysaccharide (LPS) and membrane protein profiles. All the P. piscicida strains had the same electrophoretic LPS pattern, showing O side chains with a ladder-like structure, and shared at least four major outer membrane proteins, of 20, 30, 42, and 53 kDa. Western blot (immunoblot) analysis with LPS and protein indicated that all the P. piscicida strains are immunologically related. In addition, the chromosomal DNA fingerprint patterns obtained for the European strains with the enzymes EcoRI and BamHI were practically identical to those of the Japanese and U.S. strains. Although some differences were found in the plasmid profiles of P. piscicida, a large number of strains possessed in common plasmid bands of 20 and 7 MDa. In addition, a plasmid of 50 MDa was present in the majority of the European strains. Restriction endonuclease analysis demonstrated the genetic homology of the plasmid bands shared by most of the European strains. All the P. piscicida strains had the same drug resistance patterns, indicating that a correlation between plasmid carriage and resistance to a specific antimicrobial agent cannot be established. The high levels of phenotypic, serological, and genetic homogeneity found among the P. piscicida strains should facilitate the development of DNA probes with diagnostic purposes as well as the design of effective vaccines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskaleros P. A., Stoebner J. A., Payne S. M. Iron uptake in Plesiomonas shigelloides: cloning of the genes for the heme-iron uptake system. Infect Immun. 1991 Aug;59(8):2706–2711. doi: 10.1128/iai.59.8.2706-2711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. L. O-serotyping of Yersinia ruckeri with special emphasis on European isolates. Vet Microbiol. 1990 May;22(4):299–307. doi: 10.1016/0378-1135(90)90016-o. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacre C. L., Glisson J. R. A serotypic survey of Pasteurella multocida isolated from poultry. Avian Dis. 1986 Jul-Sep;30(3):632–633. [PubMed] [Google Scholar]
- Ikeda J. S., Hirsh D. C. Antigenically related iron-regulated outer membrane proteins produced by different somatic serotypes of Pasteurella multocida. Infect Immun. 1988 Sep;56(9):2499–2502. doi: 10.1128/iai.56.9.2499-2502.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen W. A., Surgalla M. J. Morphology, physiology, and serology of a Pasteurella species pathogenic for white perch. (Roccus americanus). J Bacteriol. 1968 Nov;96(5):1606–1610. doi: 10.1128/jb.96.5.1606-1610.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike Y., Kuwahara A., Fujiwara H. Characterization of "Pasteurella" piscicida isolated from white perch and cultivated yellowtail. Jpn J Microbiol. 1975 Aug;19(4):241–247. doi: 10.1111/j.1348-0421.1975.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- SNIESZKO S. F., BULLOCK G. L., HOLLIS E., BOONE J. G. PASTEURELLA SP. FROM AN EPIZOOTIC OF WHITE PERCH (ROCCUS AMERICANUS) IN CHESAPEAKE BAY TIDEWATER AREAS. J Bacteriol. 1964 Dec;88:1814–1815. doi: 10.1128/jb.88.6.1814-1815.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen U. B., Larsen J. L. Serotyping of Vibrio anguillarum. Appl Environ Microbiol. 1986 Mar;51(3):593–597. doi: 10.1128/aem.51.3.593-597.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Colwell R. R., Hetrick F. M. Characterization of plasmids in bacterial fish pathogen. Infect Immun. 1983 Jan;39(1):184–192. doi: 10.1128/iai.39.1.184-192.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toranzo A. E., Barja J. L., Potter S. A., Colwell R. R., Hetrick F. M., Crosa J. H. Molecular factors associated with virulence of marine vibrios isolated from striped bass in Chesapeake Bay. Infect Immun. 1983 Mar;39(3):1220–1227. doi: 10.1128/iai.39.3.1220-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]