Abstract
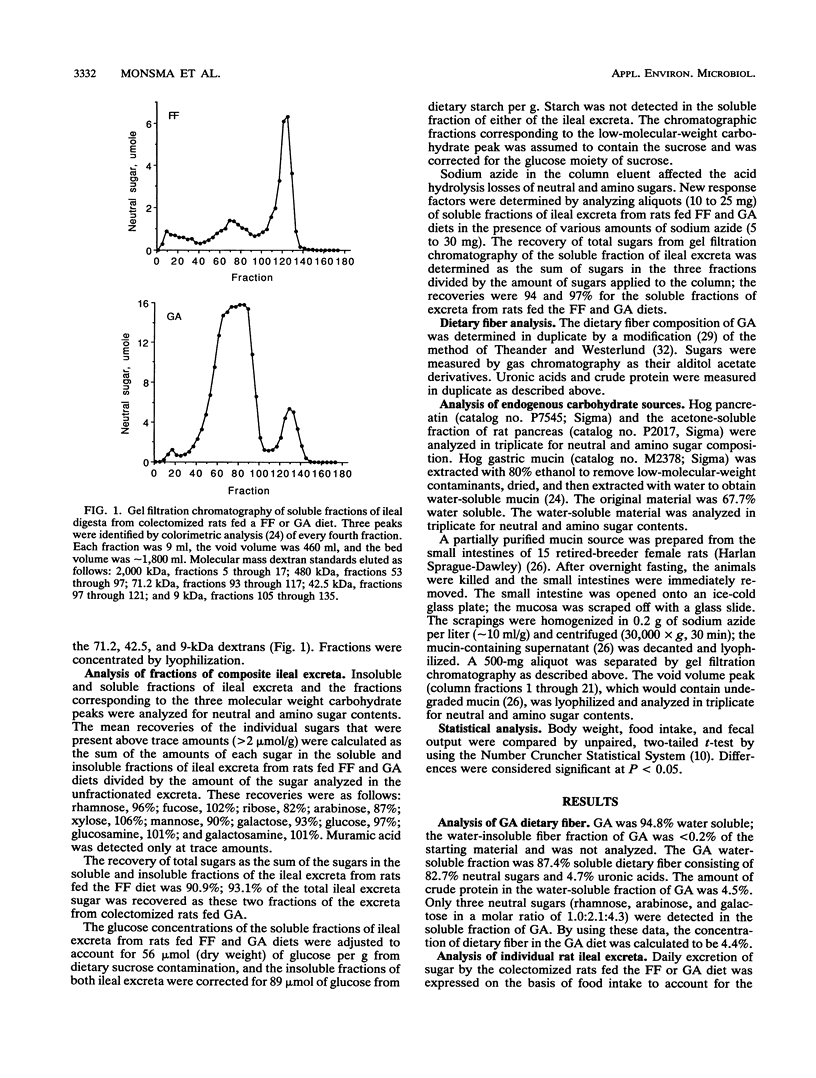
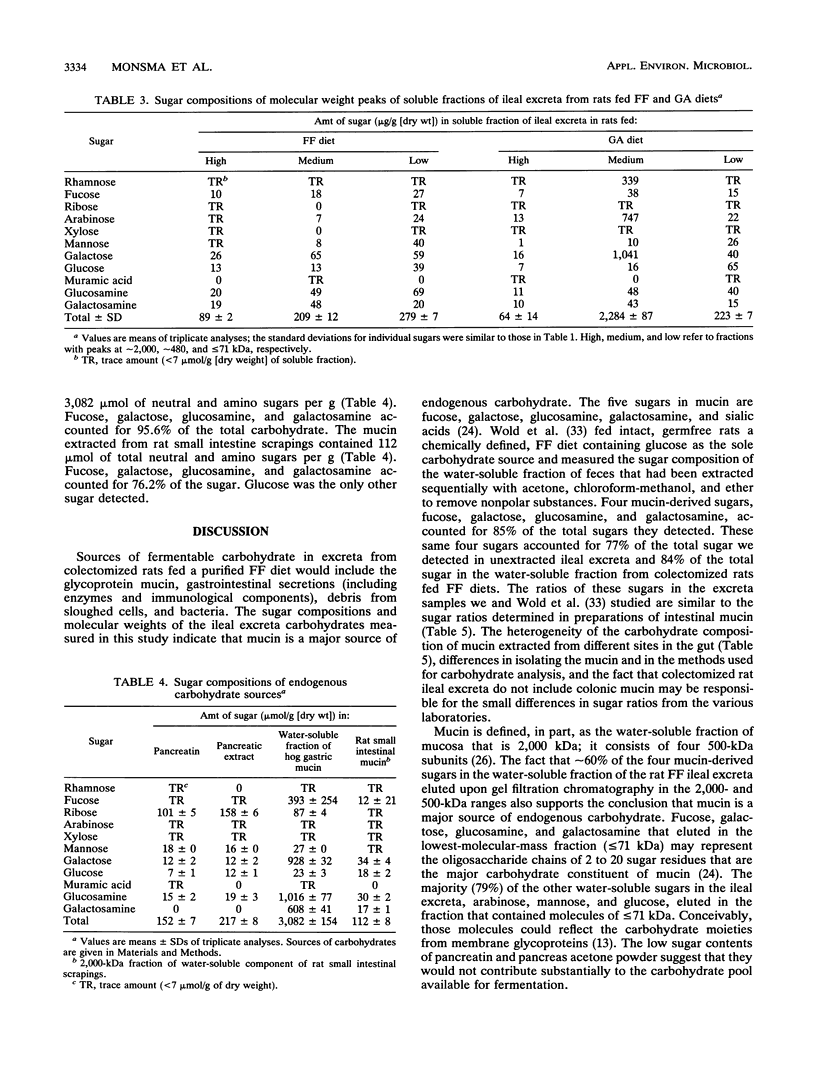
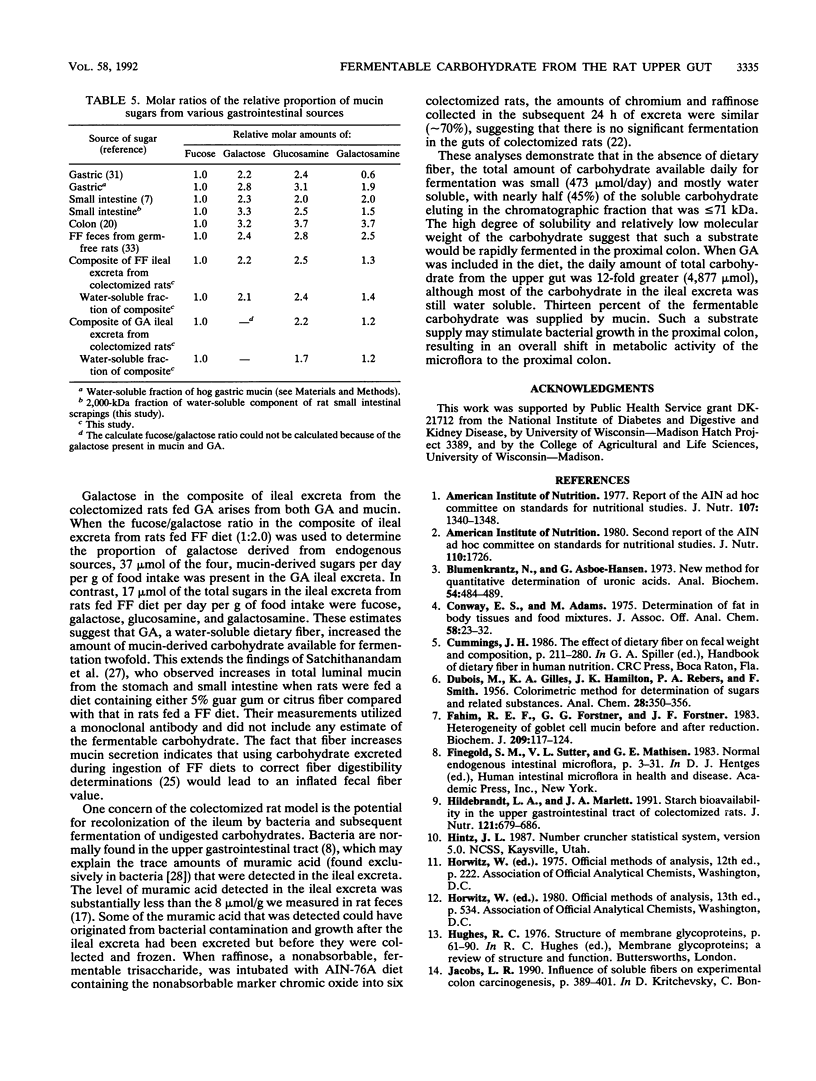
The primary aim of this study was to characterize the carbohydrate that would be supplied to the colon for fermentation under physiological conditions. Colectomized rats were fed fiber-free diets or diets containing 5% (wt/wt) gum arabic. Four (fucose, galactose, glucosamine, and galactosamine) of 11 analyzed sugars accounted for 77% of the total sugar in ileal excreta from colectomized rats fed fiber-free diets. The three sugars in gum arabic, rhamnose, arabinose, and galactose, accounted for 84% of the total sugars in gum arabic ileal excreta. Comparisons of the sugar compositions of the ileal excreta, the water-soluble fractions of the excreta, and three gel filtration fractions of the water-soluble material with those of the water-soluble fraction of rat mucosa, the acetone-soluble fraction of pancreas, and pancreatin suggested that the major source of endogenous carbohydrate is mucin. Gum arabic increased the daily excretion of the four mucin-derived sugars (fucose, galactose, glucosamine, and galactosamine) by the colectomized rats from 473 mumol per day to 634 mumol per day. We conclude that mucin is the major endogenous carbohydrate excreted from the upper gut and that gum arabic increases the amount of this endogenous carbohydrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Conway E. S., Adams M. Fats and oils. Determination of fat in body tissues and in food mixtures. J Assoc Off Anal Chem. 1975 Jan;58(1):23–27. [PubMed] [Google Scholar]
- Fahim R. E., Forstner G. G., Forstner J. F. Heterogeneity of rat goblet-cell mucin before and after reduction. Biochem J. 1983 Jan 1;209(1):117–124. doi: 10.1042/bj2090117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt L. A., Marlett J. A. Starch bioavailability in the upper gastrointestinal tract of colectomized rats. J Nutr. 1991 May;121(5):679–686. doi: 10.1093/jn/121.5.679. [DOI] [PubMed] [Google Scholar]
- Jenkins D. J., Reynolds D., Leeds A. R., Waller A. L., Cummings J. H. Hypocholesterolemic action of dietary fiber unrelated to fecal bulking effect. Am J Clin Nutr. 1979 Dec;32(12):2430–2435. doi: 10.1093/ajcn/32.12.2430. [DOI] [PubMed] [Google Scholar]
- Kraus R. J., Shinnick F. L., Marlett J. A. Simultaneous determination of neutral and amino sugars in biological materials. J Chromatogr. 1990 Jul 27;513:71–81. doi: 10.1016/s0021-9673(01)89426-7. [DOI] [PubMed] [Google Scholar]
- LaMont J. T., Ventola A. S. Purification and composition of colonic epithelial mucin. Biochim Biophys Acta. 1980 Nov 20;626(1):234–243. doi: 10.1016/0005-2795(80)90214-7. [DOI] [PubMed] [Google Scholar]
- Marlett J. A., Johnson E. J. Composition of fecal fiber from human subjects. J Nutr. 1985 May;115(5):650–660. doi: 10.1093/jn/115.5.650. [DOI] [PubMed] [Google Scholar]
- Nyman M., Asp N. G. Fermentation of dietary fibre components in the rat intestinal tract. Br J Nutr. 1982 May;47(3):357–366. doi: 10.1079/bjn19820047. [DOI] [PubMed] [Google Scholar]
- Pearson J., Allen A., Venables C. Gastric mucus: isolation and polymeric structure of the undegraded glycoprotein: its breakdown by pepsin. Gastroenterology. 1980 Apr;78(4):709–715. [PubMed] [Google Scholar]
- Satchithanandam S., Vargofcak-Apker M., Calvert R. J., Leeds A. R., Cassidy M. M. Alteration of gastrointestinal mucin by fiber feeding in rats. J Nutr. 1990 Oct;120(10):1179–1184. doi: 10.1093/jn/120.10.1179. [DOI] [PubMed] [Google Scholar]
- Sharon N. The bacterial cell wall. Sci Am. 1969 May;220(5):92–98. doi: 10.1038/scientificamerican0569-92. [DOI] [PubMed] [Google Scholar]
- Shinnick F. L., Longacre M. J., Ink S. L., Marlett J. A. Oat fiber: composition versus physiological function in rats. J Nutr. 1988 Feb;118(2):144–151. doi: 10.1093/jn/118.2.144. [DOI] [PubMed] [Google Scholar]
- Slavin J. L., Brauer P. M., Marlett J. A. Neutral detergent fiber, hemicellulose and cellulose digestibility in human subjects. J Nutr. 1981 Feb;111(2):287–297. doi: 10.1093/jn/111.2.287. [DOI] [PubMed] [Google Scholar]
- Spee-Brand R., Strous G. J., Kramer M. F. Isolation and partial characterization of rat gastric mucous glycoprotein. Biochim Biophys Acta. 1980 Jan 24;621(1):104–116. doi: 10.1016/0005-2795(80)90066-5. [DOI] [PubMed] [Google Scholar]
- Wold J. K., Khan R., Midtvedt T. Intestinal glycoproteins of germfree rats. Chemical composition of intestinal and fecal mucus from germfree rats fed a chemically defined diet. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(4):525–530. [PubMed] [Google Scholar]



