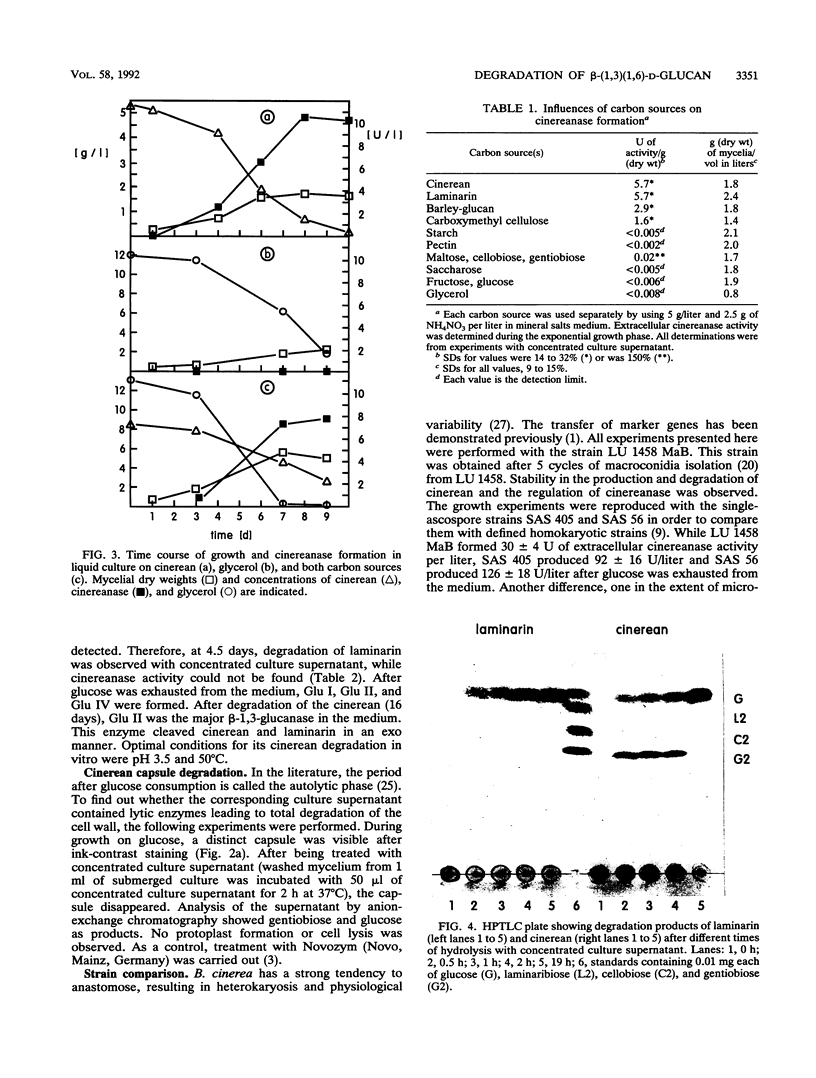
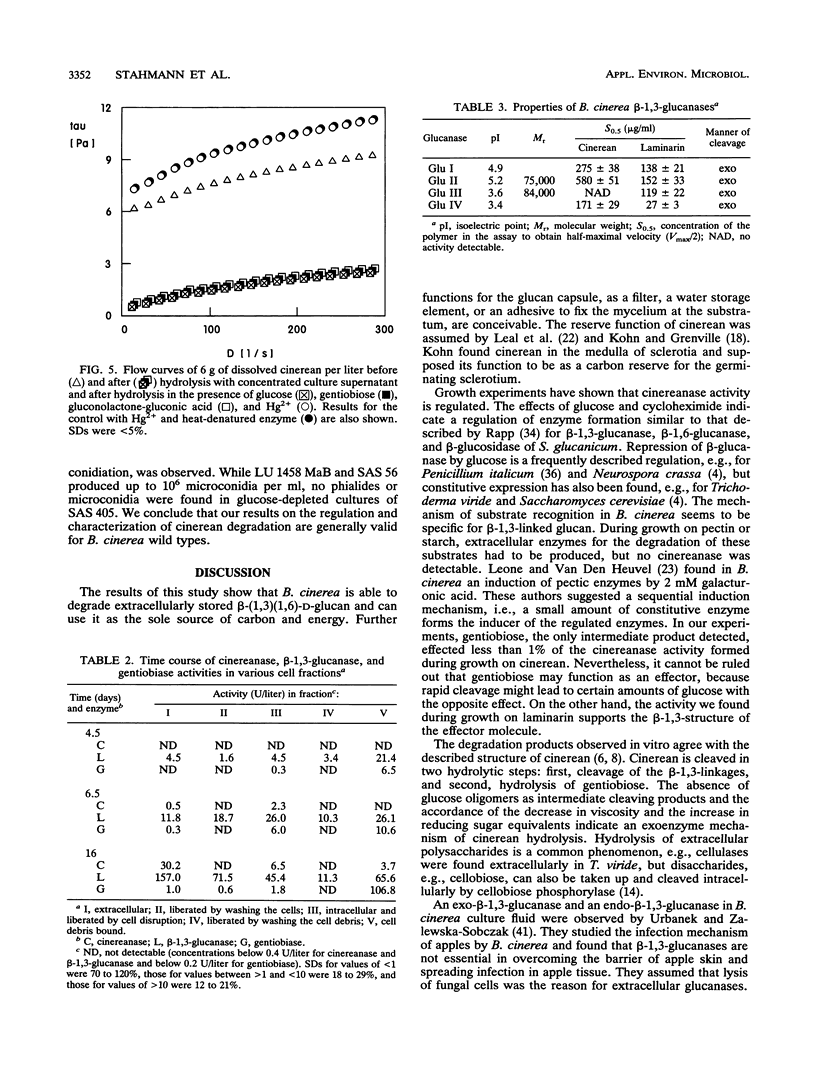
Abstract
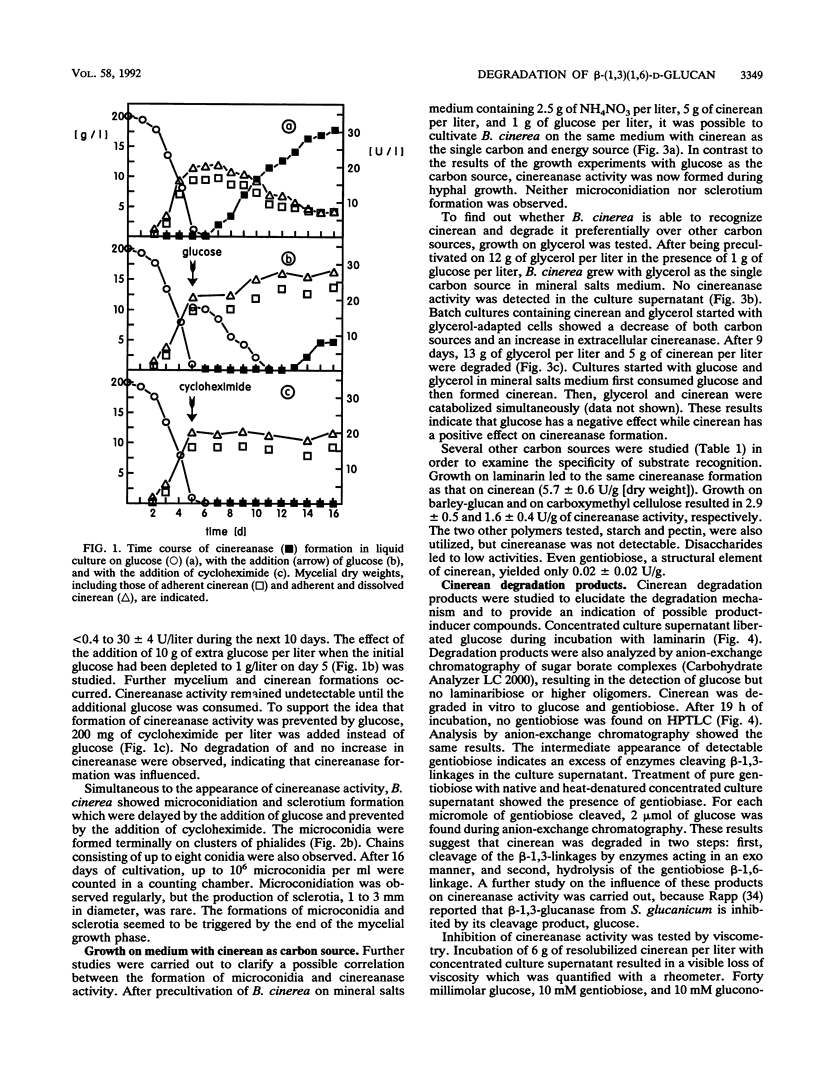
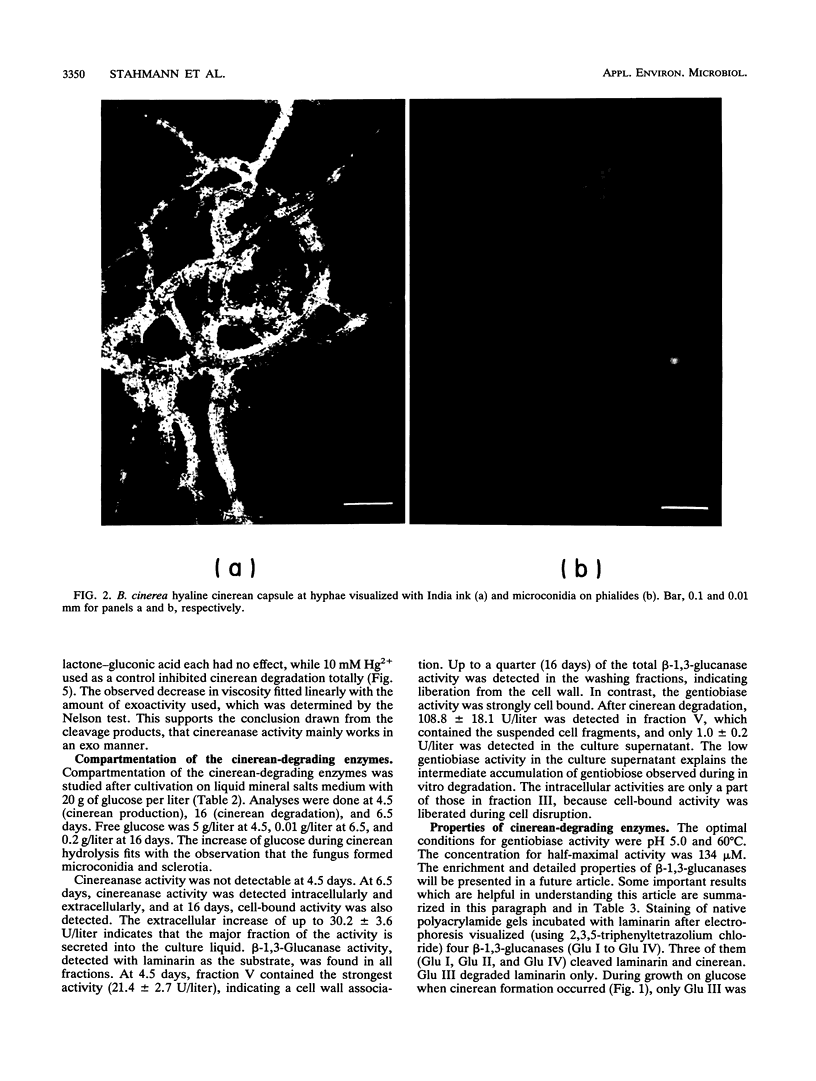
During growth on glucose, Botrytis cinerea produced extracellular β-(1,3)(1,6)-d-glucan (cinerean), which formed an adhering capsule and slime. After glucose was exhausted from the medium, cinereanase activity increased from <0.4 to 30 U/liter, effecting a striking loss in the viscosity of the culture. Cinerean was cleaved into glucose and gentiobiose. Gentiobiose was then hydrolyzed to glucose. While cinereanase activity was strongest in the culture supernatant, gentiobiase activity was located mainly in the cell wall fraction. The addition of extra glucose or cycloheximide prevented the cinerean degradation caused by an effect on cinereanase formation. Cinerean degradation was accompanied by microconidiation and sclerotium formation. B. cinerea was found to grow on cinerean with the latter as its single carbon and energy source. In this case, cinerean degradation occurred during hyphal growth, and no microconidiation or sclerotium formation was observed. Growth experiments with various carbon sources indicated that cinerean had a positive effect on the formation of cinerean-degrading enzymes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Dubourdieu D., Ribéreau-Gayon P. Mise en évidence d'une bêta-(1 à 3)-glucanase exocellulaire chez Botrytis cinera. C R Seances Acad Sci D. 1980 Jan 7;290(1):25–28. [PubMed] [Google Scholar]
- Hüttermann A., Volger C. Cellobiose phosphorylase in Fomes annosus. Nat New Biol. 1973 Sep 12;245(141):64–64. doi: 10.1038/newbio245064a0. [DOI] [PubMed] [Google Scholar]
- Jones D., Gordon A. H., Bacon J. S. Co-operative action by endo- and exo-beta-(1 leads to 3)-glucanases from parasitic fungi in the degradation of cell-wall glucans of Sclerotinia sclerotiorum (Lib.) de Bary. Biochem J. 1974 Apr;140(1):47–55. doi: 10.1042/bj1400047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Ohno N., Yadomae T. Preparation and antitumor activity of hydroxyethylated derivatives of 6-branched (1----3)-beta-D-glucan, SSG, obtained from the culture filtrate of Sclerotinia sclerotiorum IFO 9395. Chem Pharm Bull (Tokyo) 1990 Sep;38(9):2527–2531. doi: 10.1248/cpb.38.2527. [DOI] [PubMed] [Google Scholar]
- Maheshwari R., Choudari B. P. Potentiation of immune response against malaria in immunocompromised mice through glucan as an immunoadjuvant. Indian J Exp Biol. 1990 Oct;28(10):901–905. [PubMed] [Google Scholar]
- Menzinger W. Karyologische Untersuchungen an Arten und Formen der Gattung Botrytis Mich. Arch Mikrobiol. 1965 Oct 14;52(2):178–196. [PubMed] [Google Scholar]
- Misaki A., Kawaguchi K., Miyaji H., Nagae H., Hokkoku S., Kakuta M., Sasaki T. Structure of pestalotan, a highly branched (1----3)-beta-D-glucan elaborated by Pestalotia sp. 815, and the enhancement of its antitumor activity by polyol modification of the side chains. Carbohydr Res. 1984 Jul 1;129:209–227. doi: 10.1016/0008-6215(84)85313-6. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Taylor F. B., Lee D. B., Vanheuvelen W., Grossman I., Hasson H. H., Mitchell H. C., Olive D. R., Pearl E. H., Price J. W., Shull I. F. Environmental Health in Community Growth: An Administrative Guide to Metropolitan Area Sanitation Practices Committee, Engineering and Sanitarian Section, APHA. Am J Public Health Nations Health. 1963 May;53(5):802–822. doi: 10.2105/ajph.53.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]