Abstract
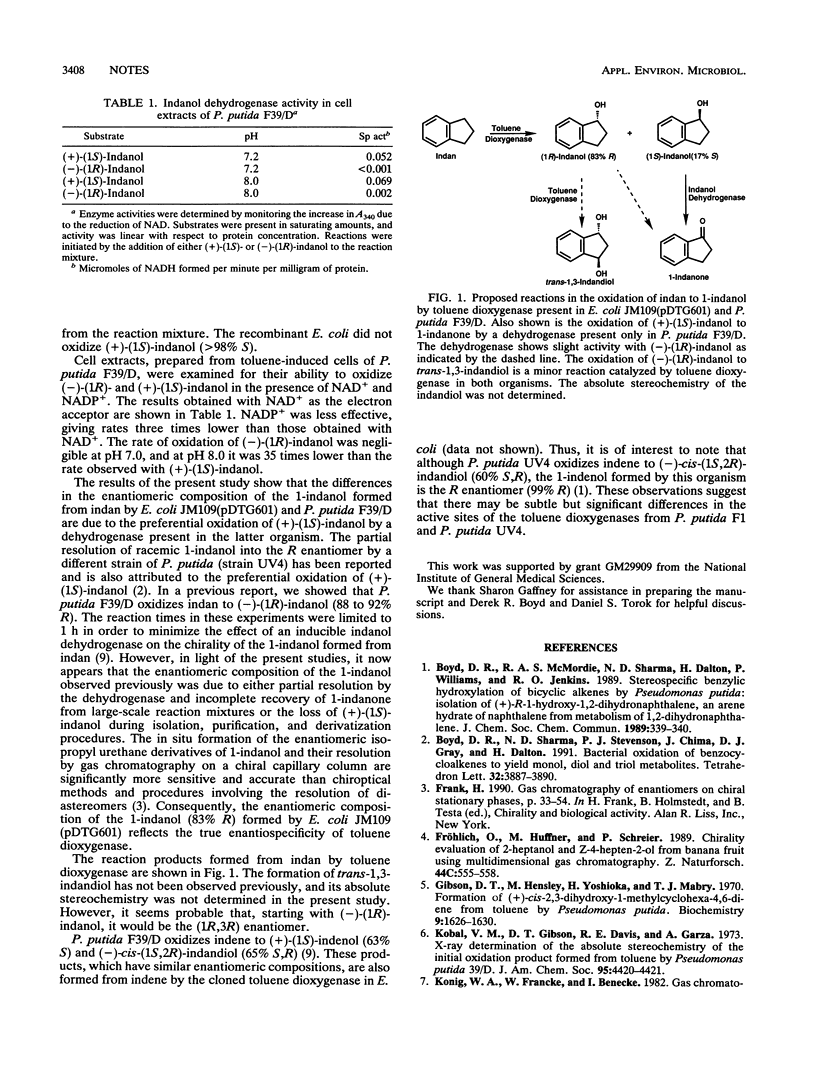
Escherichia coli JM109(pDTG601), containing the todC1C2BA genes encoding toluene dioxygenase from Pseudomonas putida F1, oxidizes indan to (-)-(1R)-indanol (83% R) and trans-1,3-indandiol. Under similar conditions, P. putida F39/D oxidizes indan to (-)-(1R)-indanol (96% R), 1-indanone, and trans-1,3-indandiol. The differences in the enantiomeric composition of the 1-indanols formed by the two organisms are due to the presence of a 1-indanol dehydrogenase in P. putida F39/D that preferentially oxidizes (+)-(1S)-indanol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Kobal V. M., Gibson D. T., Davis R. E., Garza A. X-ray determination of the absolute stereochemistry of the initial oxidation product formed from toluene by Pseudomonas puida 39-D. J Am Chem Soc. 1973 Jun 27;95(13):4420–4421. doi: 10.1021/ja00794a048. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Kwart L. D., Gibson D. T. Benzylic monooxygenation catalyzed by toluene dioxygenase from Pseudomonas putida. Biochemistry. 1988 Feb 23;27(4):1360–1367. doi: 10.1021/bi00404a041. [DOI] [PubMed] [Google Scholar]
- Zylstra G. J., Gibson D. T. Toluene degradation by Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli. J Biol Chem. 1989 Sep 5;264(25):14940–14946. [PubMed] [Google Scholar]


