Abstract
Telomerase is a ribonucleoprotein enzyme which has been linked to malignant transformation in human cells. Telomerase activity is increased in the vast majority of human tumors, making its gene product the first molecule common to all human tumors. The generation of endogenously processed telomerase peptides bound to Class I MHC molecules could therefore target cytotoxic T lymphocytes (CTL) to tumors of different origins. This could advance vaccine therapy against cancer provided that precursor CTL recognizing telomerase peptides in normal adults and cancer patients can be expanded through immunization. We demonstrate here that the majority of normal individuals and patients with prostate cancer immunized in vitro against two HLA-A2.1 restricted peptides from telomerase reverse transcriptase (hTRT) develop hTRT-specific CTL. This suggests the existence of precursor CTL for hTRT in the repertoire of normal individuals and in cancer patients. Most importantly, the CTL of cancer patients specifically lysed a variety of HLA-A2+ cancer cell lines, demonstrating immunological recognition of endogenously processed hTRT peptides. Moreover, in vivo immunization of HLA-A2.1 transgenic mice generated a specific CTL response against both hTRT peptides. Based on the induction of CTL responses in vitro and in vivo, and the susceptibility to lysis of tumor cells of various origins by hTRT CTL, we suggest that hTRT could serve as a universal cancer vaccine for humans.
Telomerase is a unique ribonucleoprotein that mediates RNA-dependent synthesis of telomeric DNA (1), the distal ends of eukaryotic chromosomes that stabilize the chromosomes during replication (2, 3). When activated, telomerase synthesizes telomeric DNA and compensates for its loss with each cell division (4). Because telomeres shorten progressively with successive cell divisions, telomere length is considered to mirror the replicative history of cell lineage (5) and cell population dynamics (6, 7). In mice, telomerase appears to play an essential role in the long-term viability of high-renewal organ systems such as the reproductive and hemopoietic systems (8).
Maintenance of a constant telomere length ensures chromosomal stability, prevents cells from aging, and confers immortality (9–11). Mice lacking telomerase RNA show that telomerase activation is a key event in malignant cell transformation (8, 12, 13). In humans, in vitro studies show that the long-term ectopic expression of telomerase reverse transcriptase (hTRT) in normal fibroblasts is sufficient for immortalization but not malignant transformation (14). However, the expression of hTRT in combination with two oncogenes (SV40 T antigen and Ras) promotes tumor transformation in normal human epithelial and fibroblast cell lines (15). These transformed cells form tumors in nude mice. Thus, although telomerase per se is not tumorigenic, it plays a direct role in oncogenesis by allowing precancerous cells to proliferate continuously and become immortal. The PCR-based telomeric repeat amplification protocol (TRAP) assay (16) reveals a stricking correlation (>80%) between high telomerase activity and tumors of different histological origins and types (17, 18). In contrast, normal tissues display little or no telomerase activity (18, 19). Therefore, telomerase expression in tumors is much greater than HER2/neu and mutated p53, which range between 30 and 50%, respectively (20, 21). From the foregoing, we reasoned that expression of hTRT in cancer cells is a likely source of peptides that, on association with MHC class I molecules, could target cytotoxic T lymphocytes (CTL) to cancer cells. An interesting analogy exists with HIV-1 reverse transcriptase, an enzyme similar to hTRT, which gives origin to peptide/MHC class I complexes that target CTL responses to virus-infected cells (22). Thus, because high telomerase activity is widespread among human tumors, hTRT could serve as a universal tumor antigen for immunotherapy and vaccine approaches.
hTRT is encoded in the genome and is in all respects a self-antigen. Consequently, CD8+ T lymphocytes with a receptor for MHC/hTRT peptide complexes are expected to be eliminated during thymic negative selection, reducing the potential precursor T cell repertoire and imposing limitations on their expansion on encounter with tumor cells in adult life. Additionally, stimulation by antigen in the absence of a second signal induces clonal anergy (23), further hampering the potential repertoire. The extent to which these events affect the normal adult repertoire, and whether or not exposure to hTRT during cancer formation has any adverse effect on the ability of cancer patients to respond, is not known. Because answering these questions is relevant to future strategies of immune intervention targeted at hTRT, we analyzed the ability of normal individuals and cancer patients to mount a CTL response in vitro against two hTRT peptides restricted by the HLA-A2 allele. Our study confirms and extends the results of Vonderheide et al. (24), which appeared as the present manuscript was being prepared.
Materials and Methods
Synthetic Peptides.
hTRT synthetic peptides p540 (540ILAKFLHWL548), p865 (865RLVDDFLLV873), and MART-1 (27AAGIGILTV35) were purchased from the Biopolymer Synthesis Center (CalTech, Pasadena, CA). Synthetic peptides 128TPPAYRPPNAPIL140 of the hepatitis B core antigen, 571YLSGANLNL579 of carcinoembryonic antigen, 476VLYR-YGSFSV485 of melanoma antigen gp100, and 476ILKEPV-HGV484 of HIV-1 reverse transcriptase were purchased from Neosystem (Strasburg, France).
Human Blood Cells.
Buffy coats from normal donors were purchased from the San Diego Blood Bank. HLA-A2+ individuals were selected by fluorescence-activated cell sorter (FACS) screening by using monoclonal antibody BB7.2. Prostate cancer patients were recruited through the Division of Urology (University of California, San Diego). Blood from these patients was obtained by venipuncture. HLA-A2+ individuals were selected by FACS screening with monoclonal antibody BB7.2. Blood collection and experiments were performed in accordance with an approved Institutional Review Board (IRB).
Tumor Cell Lines.
T2 cells were a gift of Peter Creswell (Yale University, New Haven, CT). Melanoma cell lines 624 and 1351 were the gift of John Wunderlich (National Cancer Institute, Bethesda). Prostate cancer cell lines LnCap and PC-3 were the gift from Antonella Vitiello (Robert Wood Johnson Pharmaceutical Research Institute, La Jolla, CA). Breast, colon, and lung tumor cell lines were obtained from the American Type Culture Collection.
In Vitro Immunization.
Peripheral blood mononuclear cells (PBMC) were separated by centrifugation on Ficoll-Hypaque gradients and plated in 24-well plates at 5 × 105 cells/ml per well in RPMI medium 1640 supplemented with 10% human AB+ serum, L-glutamine, and antibiotics. Autologous PBMC (stimulators) were pulsed with hTRT synthetic peptides p540 or p865 (10 μg/ml) for 3 h at 37°C. Cells were then irradiated at 5,000 rads, washed once, and added to the responder cells at a responder to stimulator ratio ranging between 1:1 and 1:4. The next day, 12 units/ml IL-2 (Chiron) and 30 units/ml IL-7 (R & D Systems) were added to the cultures. Lymphocytes were restimulated weekly with peptide-pulsed autologous adherent cells as follows. First, autologous PBMC were incubated with hTRT peptide (10 μg/ml) for 3 h at 37°C. Nonadherent cells were then removed by a gentle wash and the adherent cells were incubated with fresh medium containing the hTRT peptide (10 μg/ml) for an additional 3 h at 37°C. Second, responder cells from a previous stimulation cycle were harvested, washed, and added to the peptide-pulsed adherent cells at a concentration of 5 × 105 cells/ml (2 ml/well) in medium without peptide. Recombinant IL-2 and IL-7 were added to the cultures the next day.
In Vivo Immunization.
HHD mice were immunized s.c. at the base of the tail with 100 μg of individual hTRT peptide emulsified in incomplete Freunds' adjuvant. Half of the mice were immunized with the hTRT peptide and 140 μg of the helper peptide TPPAYRPPNAPIL, which corresponds to residues 128–140 of the hepatitis B core antigen (25).
HLA-A2.1 Binding/Stabilization Assay.
The relative avidity was measured as described (25). Briefly, T2 cells were incubated overnight at 37°C in RPMI medium 1640 supplemented with human β2-microglobulin (100 ng/ml) (Sigma) in the absence (negative control) or presence of the test peptide or the reference peptide 476ILKEPVHGV484 of HIV-1 reverse transcriptase at various final peptide concentrations (0.1–100 μM). Cells were incubated with Brefeldin A (0.5 μg/ml) for 1 h and subsequently stained with a saturating concentration of monoclonal antibody BB7.2 for 30 min at +4°C, followed by washing and a second incubation with a goat antibody to mouse Ig (Fab′)2 conjugated to FITC (Caltag, South San Francisco, CA). Cells were then washed, fixed with 1% paraformaldehyde, and analyzed in a FACs Calibur cytofluorimeter (Becton Dickinson). The mean fluorescence intensity of each concentration minus that of cells without peptide was used as an estimate of peptide binding. Results are expressed as values of relative avidity (RA), which is the ratio of the concentration of test peptide necessary to reach 20% of the maximal binding by the reference peptide over that of the reference peptide, so that the lower the value the stronger the binding. Dissociation of the test peptide from the HLA-A2.1 molecule reflects the half-life of fluorescence intensity of the peptide/MHC complex over time. The half-life of the complex refers to the time (h) required for a 50% reduction of the T0 mean fluorescence intensity (25). Synthetic peptides 571YLSGANLNL579 of carcinoembryonic antigen and 476VLYRYGSFSV485 of melanoma antigen gp100 were used as internal controls to account for intertest variability and consistency with reported measures of RA and the half-life of the complex (25).
Cytotoxicity Assay.
The induction of CTL in human PBMC was monitored in a conventional 51Cr-labeling release assay. Briefly, peptide-pulsed TAP−/HLA-A2.1+ human T2 cells were incubated with 10 μg of hTRT peptides or the MART-1 control peptide for 90 min during labeling with 51Cr. After washing, the target cells were added to serially diluted effectors in 96-well microplates. After a 6-h incubation at 37°C, supernatants were harvested and counted in a Trilux Betaplate counter (Wallac, Turku, Finland). Results are expressed as the percentage of specific lysis and determined as follows: [(experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm)] × 100.
The induction of CTL in HHD mice was assessed as follows. Spleen cells were harvested 7 days after immunization and were restimulated in vitro with the corresponding hTRT peptide and 25 μg/ml lipopolysaccharide-stimulated irradiated (5,000 rads) syngeneic spleen cells. After six days of culture, the cells were harvested and tested for their ability to lyse HHD-transfected/TAP- RMA-S cells in a 4-h 51Cr-labeling release assay (25). Specific lysis was calculated as indicated in the legend of Fig. 1. Values refer to maximal cytotoxicity measured for individual responder mice at an effector-to-target ratio of 60:1.
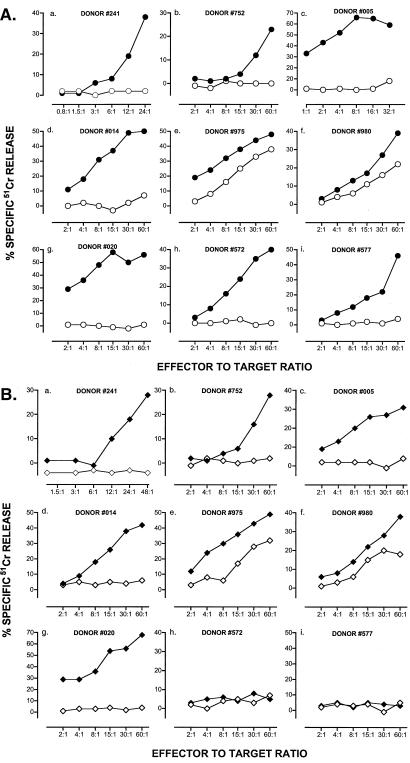
Figure 1.
Induction of CTL against hTRT in PBMC from normal blood donors. T cells from HLA-A2+ individuals were stimulated by autologous PBMC pulsed with hTRT-derived synthetic peptides as detailed in Materials and Methods. (A) Results refer to effector cells from individual donors immunized in vitro against p540. (○) T2 cells and (●) T2 cells pulsed with p540 as targets. (B) Results refer to effector cells from individual donors immunized in vitro against p865. (⋄) T2 cells and (♦) T2 cells pulsed with p865 as targets. Effector-to-target ratios are indicated on an individual basis. Percent cytotoxicity was calculated as specified in Materials and Methods.
Results
Identification and Analysis of HLA-A2.1-Restricted hTRT Peptides.
The aa sequence of hTRT (locus AF015950) (19) was analyzed for 9 mer peptide sequences containing known binding motifs for the HLA-A2.1 molecule (26), a subtype encompassing 95% of the HLA-A2 allele which is expressed in about 50% of the Caucasian population (27–29). Peptides were identified by reverse genetics based on canonical anchor residues for HLA-A2.1 (29), and by using the software of the Bioinformatics & Molecular Analysis Section (National Institutes of Health, Washington, DC) available at http://bimas.dcrt.nih.gov/molbio/hla_bind/index.html which ranks 9 mer peptides on a predicted half-time dissociation coefficient from HLA class I molecules (30). From an initial panel of ≈30 candidate peptides, we retained two sequences, 540ILAKFLHWL548 and 865RLVDDFLLV873, denoted here as p540 and p865.
Because the immunogenicity of MHC class I-restricted peptides reflects to some degree their binding and stabilizing capacity for MHC class I molecules (31–33), we sought direct proof of the strength of interaction between the two hTRT peptides and the HLA-A2.1 molecule in a conventional binding/stabilization assay that uses the antigen-transporting deficient (TAP−) HLA-A2.1+ human T2 cells. The RA calculated in reference to 476ILKEPVHGV484 of HIV-1 reverse transcriptase, a canonical high-binder peptide (25), was 2.9 and 2.5 for p540 and p865, respectively. The stability of each peptide bound to HLA-A2.1 was measured as the half-life of the complex and was in the order of 4–6 h for p540 and 2–4 h for p865. Collectively, these measurements indicate that both hTRT peptides are excellent binders to HLA-A2.1, albeit p865 has a faster dissociation rate.
CTL Response Against hTRT in Normal Human Individuals.
The presence of precursor T cells for both hTRT peptides and their expansion on antigen stimulation were tested by using PBMC of 10 HLA-A2+ normal blood donors in an in vitro immunization assay. Nine out of ten individuals responded to immunization, generating T cells that lysed peptide-pulsed T2 cells as targets starting from the third round of peptide stimulation. All nine responders generated CTL specific for p540 and seven responded against p865 (Fig. 1 A and B). The values of maximal lysis varied from individual to individual and ranged between 28–68% and 20–68%, respectively. In two instances (donor 975 and 980), there was a lower but measurable nonspecific lysis, possibly because of contaminant NK cells. Thus, by random testing of normal HLA-A2+ individuals, it was clearly established that both hTRT peptides are immunogenic, implying that precursor CTL for hTRT are present in the peripheral adult repertoire.
CTL Response Against hTRT in Cancer Patients.
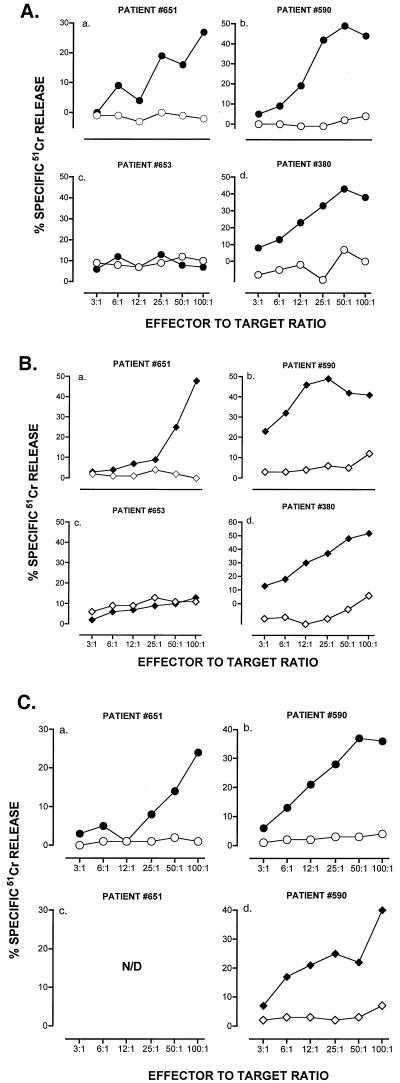
Whether or not CTL against hTRT could also be induced in cancer patients was studied in four HLA-A2.1+ individuals with clinical and histological diagnosis of prostate cancer. All four patients were refractory to hormonal therapy; three had metastases and none had prostatectomy. In prostate cancer, the most common cause of cancer in men, high hTRT expression has been documented in 84% of cases (34). Marked lysis of peptide-pulsed T2 cells was observed in three out of four individuals after three rounds of in vitro stimulation (Fig. 2 A and B). Both peptides yielded comparable CTL responses in all three individuals with maximal lysis ranging between 27–49% and 48–52%, respectively. CTL against both peptides lysed LnCap, a HLA-A2.1+ prostate cancer cell line, with maximal lysis ranging between 24–36% for p540 and 12–40% for p865. Prostate cancer cell line PC-3, which is HLA-A2.1−, was used as control and was not lysed (Fig. 2C). Both prostate cancer cell lines tested positive for hTRT by the TRAPeze (telomerase detection assay; Intergen, Purchase, NY; not shown), suggesting that the CTL generated against the synthetic peptides might lyse cancer cells by recognizing hTRT peptide/MHC class I complex at the surface of cancer cells.
Figure 2.
Induction of CTL against hTRT in PBMC from prostate cancer patients. (A). Results refer to effector cells from individual patients immunized against p540. Values refer to cells tested after three rounds of in vitro stimulation. (○) T2 cells and (●) T2 cells pulsed with p540 as targets. (B). Results refer to effector cells from individual patients immunized against p865. (⋄) T2 cells and (♦) T2 cells pulsed with p865 as targets. Effector-to-target ratios are indicated on an individual basis. (C). Results refer to effector cells from individual patients immunized in vitro against p540 (circles) or p865 (diamonds). Open symbols define the HLA-A2− PC-3 prostate cancer cell line as a target. Closed symbols define the HLA-A2+ prostate cancer cell line LnCap as a target. Percent cytotoxicity was calculated as specified in Material and Methods.
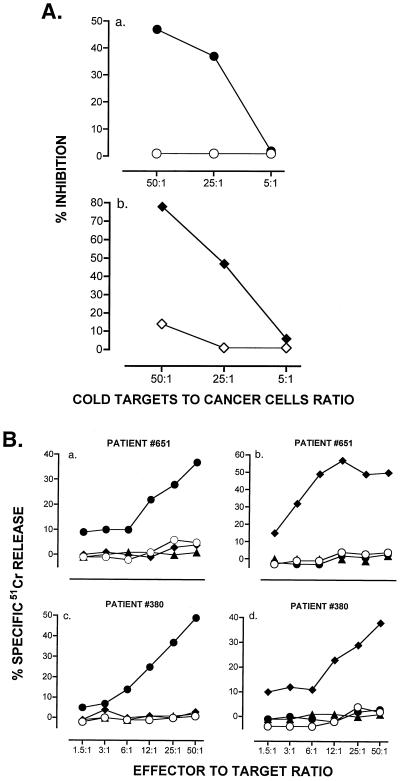
Cold target competition experiments were performed in an attempt to understand if lysis of the LnCap tumor cell line was specific for endogenously processed hTRT peptides. In these experiments, the lysis of LnCap cells by CTL from a prostate cancer patient was competed for by T2 cells pulsed in vitro with p540 or p865 (10 μg/ml). Peptide-loaded T2 cells caused a dose-dependent inhibition of lysis of LnCap cells in both peptide combinations (Fig. 3A). We further assessed the specificity of the CTL generated against each one of the two hTRT peptides by testing them on T2 targets pulsed with irrelevant HLA-A2 binding peptides. Neither T2 cells pulsed with peptide 27AAGIGILTV35 from the melanoma antigen MART-1 nor T2 cells pulsed with a nonhomologous hTRT peptide were lysed (Fig. 3B). Collectively, these studies show that (i) patients' CTL are specific for the hTRT peptide used to induce them, and (ii) lysis of prostate cancer cells is mediated by, and is specific for, endogenously processed hTRT peptides complexed with HLA-A2.1 molecules, suggesting chemical identity between naturally processed peptides on tumor cells and the synthetic peptides used for immunization. Formal validation will require elution of peptides from tumor cells and their analysis by tandem mass spectrometry (35).
Figure 3.
Molecular specificity of target recognition by CTL generated against hTRT peptides. (A). Cold target inhibition. 51Cr-labeled LnCap cells (5 × 104 cells/ml) were mixed with T2 cells (open symbols) or T2 cells pulsed with p540 (●) or p865 (♦) (1 μg/ml) at a cold-to-hot target cell ratio of 5:1, 25:1, and 50:1. CTL lines 380.540.1 and 380.865.1 of patient no. 380 generated against p540 and p865, respectively, were added at an effector-to-target ratio of 50:1. (B) Lysis of T2 cells pulsed with irrelevant HLA-A2 binding peptides. Results refer to lysis by CTL of patient (no. 651) generated against p540 (a) or p865 (b), and CTL of patient (no. 380) generated against p540 (c) or p865 (d). Closed symbols define T2 cells pulsed with p540 (circles), p865 (diamonds), and MART-1 peptide (triangles). (○) Nonpulsed T2 cells. Percent cytotoxicity was calculated as specified in Materials and Methods.
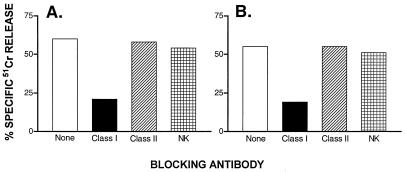
Studies on MHC restriction were performed by using blocking antibodies. Lysis of peptide-pulsed T2 cells by CTL lines generated from a prostate cancer patient was inhibited by the anti-MHC class I monoclonal antibody BB7.2 in both peptide combinations (Fig. 4), but not by the anti-MHC class II monoclonal antibody Q5/13 (36) nor by transfectoma antibody γ1RGD3 that blocks NK cells (37). By two-color FACS analysis, the phenotype of T cells proliferating after three rounds of in vitro stimulation with hTRT peptide was CD3+ (78%), CD8+ (37%), CD4+ (36%), and CD16/56 (6%). Collectively, these experiments confirm that effector T cells generated by in vitro immunization are MHC class I-restricted (CD8+) T cells which do not possess NK activity.
Figure 4.
CTL of prostate cancer patient against hTRT are MHC Class I restricted. CTL lines 380.540.1 and 380.865.1 of a patient (no. 380) were tested in a 51Cr-labeled release assay by using as targets T2 cells pulsed with p540 (A) or p865 (B). The following inhibitory antibodies were used: murine monoclonal antibody BB7.2 (IgG2b) against MHC Class I, murine monoclonal antibody Q5/13 (IgG2a) against HLA-DR, and the engineered antibody γ1RGD3 that blocks NK cell function.
hTRT is expressed in normal cells such as circulating B and T cells, germinal center B cells, thymocytes, and CD34+ progenitor hemopoietic cells (6, 7, 38). This implies that CTL generated against hTRT peptides could engender an autoimmune attack on normal cells. To this end, we verified whether the CTL of a cancer patient would lyse HLA-A2+ CD34+ cells. Neither CTL against p540 nor those against p865 induced any lysis over a wide range of effector-to-target ratios (not shown). Thus, at least with respect to hemopoietic stem cells, an autoimmune attack appears unlikely. This is consistent with the fact that activated T cells were not lysed by hTRT CTL in culture.
CTL Response Against hTRT in HLA-A2.1-Transgenic Mice.
Whether peptides can serve as immunogens in vivo and elicit a CTL response depends on a variety of factors such as the mode of immunization, suitable activation of antigen-presenting cells, the frequency of precursor cells, and binding and stabilization of MHC class I molecules by peptide. In this study, we demonstrated (Table 1) that both peptides bind to HLA-A2.1 with a RA <3 but possess different dissociation rates. In either case, we were able to generate CTL responses in vitro from PBMC of normal blood donors as well as prostate cancer patients. Therefore, a reasonable expectation would be that they may also be immunogenic in vivo. To test this possibility, we immunized H-2Db−/−, β2m−/−, and HLA-A2.1+ monochain transgenic (HHD) mice (39). In these mice, the peripheral CD8+ T cell repertoire is essentially educated on the transgenic human molecule. Therefore, HHD mice are an excellent tool to assess at the preclinical level the ability of individual peptides to induce HLA-A2.1-restricted CTL responses in vivo (25).
Table 1.
Induction of CTL against hTRT in HLA-A2.1 transgenic mice
| Group | hTRT peptide | Helper peptide | No. of responders | Percent lysis |
|---|---|---|---|---|
| 1 | 540ILAKFLHWL548 | − | 10 /15 (66%) | (35, 21, 34, 42, 56, 21, 12, 35, 42, 16) |
| 2 | " | + | 8 /10 (80%) | (45, 56, 62, 64, 65, 45, 65, 45) |
| 3 | 865RLVDDFLLV873 | − | 3 /15 (20%) | (25, 12, 15) |
| 4 | " | + | 7 /10 (70%) | (25, 32, 35, 12, 16, 18, 21) |
HHD mice were immunized by a s.c. injection of 100 μg of hTRT peptide emulsified in incomplete Freunds' adjuvant. In Groups 2 and 4, the hTRT peptide was administered together with 140 μg of the helper peptide TPPAYRPPNAPIL (25). Values of cytotoxicity refer to individual responder mice. Spleen-derived CTL were harvested 7 days after immunization and then cultured for 6 days with the homologous hTRT peptide. Values refer to maximal cytotoxicity at an effector-to-target ratio of 60∶1.
Both p540 and p865 were able to induce specific CTL responses (Table 1) although differences were noted. In fact, p540 induced CTL whether alone or in combination with a helper peptide (66 vs. 80% responders). In contrast, a high (70%) response against p865 was obtained only when its immunogenicity was increased by association with the helper peptide. The different immunogenicity of the two hTRT peptides was also reflected by the magnitude of individual responses (55.8 ± 9.4 vs. 20 ± 11.5% lysis) against p540 and p865 with helper peptide, respectively. This is consistent with the observation that two normal blood donors responded to immunization against p540 but failed to respond against p865 (Fig. 1). Thus, there is an overall correlation between the results of binding/stabilization of the HLA-A2.1 molecule, the results of immunogenicity in vitro of human PBMC, and the response in vivo in HHD mice. Finally, to exclude the development of untoward autoimmunity, HHD mice immunized against hTRT peptides were monitored with respect to the number of circulating B lymphocytes. By using a dual stain (B220 and anti-Ig) FACS analysis, we found no decrease in circulating B cells in immunized mice when compared with normal HHD mice (not shown). Furthermore, no enlarged mesenteric lymph nodes nor cellular infiltrates in the liver were noticed after immunization (not shown).
CTL of Cancer Patient Kill Tumor Cells of Various Origins and Types.
Because CTL generated against p540 and p865 recognize naturally processed hTRT peptides on LnCap prostate cancer cells and hTRT activity is expressed at high levels in the vast majority of human cancers, recognition of hTRT-derived peptides by CTL could mediate killing of a wide variety of cancer types. CTL lines from a prostate cancer patient were used in a 51Cr-labeled release assay to assess lysis of HLA-A2+ tumor cell lines of breast, colon, lung, and melanoma origin as targets. By the TRAPeze assay, all these cell lines were hTRT positive. Peptide-pulsed T2 cells and the LnCap prostate cancer cell line served as positive controls (Table 2). All cell lines but the SW480 colon cell line were lysed by CTL generated against p540 (range lysis 39–48%). On the other hand, all cell lines but the H69 lung cell line were lysed by CTL generated against p865 (range lysis 37–41%). The cytotoxic activity was dependent on expression of the HLA-A2 molecule because tumor-matched cell lines of a different HLA type were not lysed. Collectively, these data indicate that hTRT peptides such as p540 and p865 are naturally processed in a variety of tumor cell types.
Table 2.
CTL of cancer patient kill tumor cells of various origins and types
| Cell target | Tumor origin | hTRT expression* | HLA-A2† | Percent lysis‡
|
|
|---|---|---|---|---|---|
| CTL p540§ | CTL p865 | ||||
| T2 + peptide | ND | Pos. | 59 | 48 | |
| T2 | ND | Pos. | 11 | 4 | |
| MCF7 | Breast | Pos. | Pos. | 39 | 41 |
| SKBR3 | Pos. | Neg. | 7 | 9 | |
| SW480 | Colon | Pos. | Pos. | 12 | 37 |
| HCT011 | Pos. | Neg. | 9 | 6 | |
| H69 | Lung | Pos. | Pos. | 41 | 9 |
| H146 | Pos. | Neg. | 11 | 5 | |
| 624 | Melanoma | Pos. | Pos. | 48 | 39 |
| 1351 | Pos. | Neg. | 12 | 6 | |
| LnCap | Prostate | Pos. | Pos. | 44 | 41 |
| Pc3 | Pos. | Neg. | 9 | 5 | |
Pos., positive; Neg., negative.
hTRT expression of the tumor cells was determined by a PCR-based assay (TRAPeze, Intergen, Purchase, NY).
† Expression of HLA-A2 was measured by flow cytometry with the monoclonal antibody BB7.2.
‡ Cellular cytotoxicity was measured in a 51Cr-labeled release assay at an effector-to-target ratio of 50∶1. All tumor cell lines were incubated with 100 units/ml of recombinant IFN-γ for 48 h before the 51Cr-labeled release assay.
§ CTL lines 380.540.1 and 380.865.1 of patient were generated by immunization with p540 and p865, respectively.
Discussion
We demonstrate that hTRT peptides can expand precursor CTL in PBMC of normal individuals and patients with prostate cancer, and induce in both instances MHC class I-restricted, peptide-specific CTL responses. Therefore, the first major implication from our findings is that the available CTL repertoire for hTRT is similarly preserved not only in normal individuals as recently reported (24) but also, and more importantly, in individuals with cancer. This suggests that exposure to cancer does not cause deletion or anergy of clonotypes specific for hTRT. Because the three patients responding to immunization were resistant to hormone therapy and had metastases, it was surprising that hTRT CTL could be induced at such an advanced stage of disease generally characterized by immunosuppression. Based on these considerations, one could predict that because the frequency of precursors from PBMC is high enough to permit their expansion in vitro and because hTRT peptides bind to MHC class I with sufficient avidity, the two peptides identified in this study may be used for vaccination of HLA-A2+ cancer patients.
The finding that the CTL of a prostate cancer patient mediate efficient lysis of a variety of HLA-A2+ cancer cells such as prostate, breast, colon, lung, and melanoma is unprecedented. Based on the values of specific lysis, it appears as if these cancer cells are equally effective in processing and presenting the same endogenous hTRT peptides. Therefore, a second major implication of our study is that similar hTRT peptides are expressed and complexed with MHC class I molecules on a variety of cancer cells of different histological origins and types. This renders them susceptible to destruction by CTL and underscores the potential advantage hTRT immunization may have in the control of primary tumors and metastases in a large variety of cancer types in humans.
The in vivo immunogenicity of hTRT peptides in patients with cancer and the clinical efficacy of a possible hTRT-based vaccination cannot be accurately predicted because many factors, alone or in combination, can influence the outcome of these events. These include the efficacy of methods used to deliver the vaccine and trigger immunity, limitations in the T cell repertoire for the peptide used, and the down-regulation of MHC (40, 41) and TAP (42, 43) molecules observed in cancer cells. The mixed results of recent clinical trials in patients vaccinated with melanoma-associated peptides administered with immunological adjuvant (44) or autologous dendritic cells (45) underscore these concerns. Thus, the success of telomerase-based vaccines in cancer patients will hinge on effective methods to elicit a CTL response avoiding a selective pressure against single hTRT epitopes [e.g., by using polyepitope vaccines (25, 46)], and improve antigen presentation by tumor cells even though CTL kill susceptible targets that display an extremely low number of MHC-peptide complexes at their surface (47).
The future of hTRT-based vaccination will also depend on the type of side effects that may follow immunization. Because hTRT is expressed in stem cells and mature hemopoietic cells (6, 7, 38), the possibility exists that hTRT vaccination could result in autoimmunity and destruction of normal cells. In our hands, the CTL of a cancer patient specific for either p540 or p865 failed to lyse HLA-A2+ CD34+ cells. Similarly, CTL against p540 raised in normal individuals did not lyse HLA-A2+ CD34+ cells (24). Together with the lack of overt autoimmune defects in hemopoietic cells and in the liver in HHD mice after vaccination with hTRT peptides, we provisionally conclude that CTL specific for hTRT are unlikely to trigger autoimmunity against normal cells. Possibly, the quantity of hTRT peptides generated under physiological lineage/clonotype activation and differentiation is insufficient to mediate lysis by CTL. Whether the same holds true for germ cells of reproductive organs for which little is known about CD8 T cell-mediated autoimmunity, can only be speculated. Whereas additional experiments are needed, the fact that autoimmunity does not develop after immunization against tumor antigens shared by normal tissues (48, 49), including the lymphoid tissue (50) and reproductive organs (51), supports the view that hTRT-based vaccination in cancer patients may be possible and safe.
In conclusion, based on the demonstration that precursor CTL specific for two hTRT peptides can be expanded in patients with cancer, their CTL recognize the same hTRT peptides on tumor cells of various origins and histological types, and a strong in vivo CTL response against both hTRT peptides was induced in HLA-A2.1+ monochain transgenic mice, we suggest that hTRT can be regarded as a universal cancer antigen and its peptides as the substrate for a possible universal cancer vaccine for humans.
Acknowledgments
We thank the numerous colleagues at the University of California, San Diego and neighboring institutions for the generous supply of reagents and helpful criticisms. This work was supported by an institutional grant to M.Z. and Grant M01 RROO827 to the Clinical Research Center.
Abbreviations
- hTRT
human telomerase reverse transcriptase
- PBMC
peripheral blood mononuclear cells
- CTL
cytotoxic T lymphocytes
- FACS
fluorescence-activated cell sorter
- HHD
mice transgenic for the human HLA.A2.1 molecule and double knock-out for murine H-2Db and β2microglobulin
- HLA
human leukocyte antigen
- NK
natural killer (cells)
- TAP
transporter associated protein
- TRAP
telomeric repeat amplification protocol
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070560797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070560797
References
- 1.Blackburn E H. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn E H. Nature (London) 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 3.Greider C W. Curr Opin Genet Dev. 1994;4:203–211. doi: 10.1016/s0959-437x(05)80046-2. [DOI] [PubMed] [Google Scholar]
- 4.Counter C M, Avilion A A, LeFeuvre C E, Stewart N G, Greider C W, Harley C B, Bacchetti S. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchkovich K J, Greider C W. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng N P, Levine B L, June C H, Hodes R J. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng N P, Granger L, Hodes R J. Proc Natl Acad Sci USA. 1997;94:10827–10832. doi: 10.1073/pnas.94.20.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H W, Blasco M A, Gottlieb G J, Horner J W, II, Greider C W, DePinho R A. Nature (London) 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 9.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 10.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, Caddle S D, Ziaugra L, Beijersbergen R L, Davidoff M J, Liu Q, et al. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 11.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph K L, Chang S, Lee H W, Blasco M, Gottlieb G J, Greider C, DePinho R A. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg R A, Chin L, Femino A, Lee K H, Gottlieb G J, Singer R H, Greider C W, DePinho R A. Cell. 1999;97:515–525. doi: 10.1016/s0092-8674(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 14.Morales C P, Holt S E, Ouellette M, Kaur K J, Yan Y, Wilson K S, White M A, Wright W E, Shay J W. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- 15.Hahn W C, Counter C M, Lundberg A S, Beijersbergen R L, Brooks M W, Weinberg R A. Nature (London) 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 16.Broccoli D, Young J W, de Lange T. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shay J W, Bacchetti S. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim N W. Eur J Cancer. 1997;33:781–786. doi: 10.1016/S0959-8049(97)00057-9. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 20.Marx J. Science. 1993;262:1644–1645. doi: 10.1126/science.8259506. [DOI] [PubMed] [Google Scholar]
- 21.Disis M L, Cheever M A. Adv Cancer Res. 1997;71:343–371. doi: 10.1016/s0065-230x(08)60103-7. [DOI] [PubMed] [Google Scholar]
- 22.Walker B D, Flexner C, Paradis T J, Fuller T C, Hirsch M S, Schooley R T, Moss B. Science. 1988;240:64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz R H. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 24.Vonderheide R H, Hahn W C, Schultze J L, Nadler L M. Immunity. 1999;10:673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 25.Firat H, Garcia-Pons F, Tourdot S, Pascolo S, Scardino A, Garcia Z, Michel M-L, Jack R, Jung G, Kostmatopoulos K, et al. Eur J Immunol. 1999;29:3112–3121. doi: 10.1002/(SICI)1521-4141(199910)29:10<3112::AID-IMMU3112>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Ruppert J, Sidney J, Celis E, Kubo R T, Grey H M, Sette A. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 27.Lee T D. In: The HLA System. Lee J, editor. New York: Springer; 1990. pp. 141–178. [Google Scholar]
- 28.Fernandez-Vina M A, Falco M, Sun Y, Stastny P. Hum Immunol. 1992;33:163–173. doi: 10.1016/0198-8859(92)90068-x. [DOI] [PubMed] [Google Scholar]
- 29.Krausa P, Brywka M, III, Savage D, Hui K M, Bunce M, Ngai J L, Teo D L, Ong Y W, Barouch D, Allsop C E, et al. Tissue Antigens. 1995;45:223–231. doi: 10.1111/j.1399-0039.1995.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 30.Parker K C, Bednarek M A, Coligan J E. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 31.Vitiello A, Marchesini D, Furze J, Sherman L A, Chesnut R W. J Exp Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast W M, Melief C J, Oseroff C, Yuan L, Ruppert J, et al. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 33.van der Burg S H, Visseren M J, Brandt R M, Kast W M, Melief C J. J Immunol. 1996;156:3308–3314. [PubMed] [Google Scholar]
- 34.Sommerfeld H J, Meeker A K, Piatyszek M A, Bova G S, Shay J W, Coffey D S. Cancer Res. 1996;56:218–222. [PubMed] [Google Scholar]
- 35.Hunt D F, Henderson R A, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox A L, Appella E, Engelhard V H. Science. 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 36.Quaranta V, Zanetti M, Reisfeld R A. J Exp Med. 1982;156:1551–1556. doi: 10.1084/jem.156.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zanetti M, Filaci G, Lee R H, del Guercio P, Rossi F, Bacchetta R, Stevenson F, Barnaba V, Billetta R. EMBO J. 1993;12:4375–4384. doi: 10.1002/j.1460-2075.1993.tb06122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek M A, Shay J W, Ishioka S, Yamakido M. J Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- 39.Pascolo S, Bervas N, Ure J M, Smith A G, Lemonnier F A, Perarnau B. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle A, Martin W J, Funa K, Gazdar A, Carney D, Martin S E, Linnoila I, Cuttitta F, Mulshine J, Bunn P, et al. J Exp Med. 1985;161:1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Momburg F, Degener T, Bacchus E, Moldenhauer G, Heammerling G J, Meoller P. Int J Cancer. 1986;37:179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- 42.Restifo N P, Esquivel F, Kawakami Y, Yewdell J W, Mule J J, Rosenberg S A, Bennink J R. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cromme F V, Airey J, Heemels M T, Ploegh H L, Keating P J, Stern P L, Meijer C J, Walboomers J M. J Exp Med. 1994;179:335–340. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg S A, Yang J C, Schwartzentruber D J, Hwu P, Marincola F M, Topalian S L, Restifo N P, Dudley M E, Schwarz S L, Spiess P J, et al. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nestle F O, Alijagic S, Gilliet M, Sun M, Grabbe S, Dummer R, Burg G, Schadendorf D. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 46.Thomson S A, Sherritt M A, Medveczky J, Elliott S L, Moss D J, Fernando G J, Brown L E, Suhrbier A. J Immunol. 1998;160:1717–1723. [PubMed] [Google Scholar]
- 47.Sykulev Y, Joo M, Vturina I, Tsomides T J, Eisen H N. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 48.Morgan D J, Kreuwel H T, Fleck S, Levitsky H I, Pardoll D M, Sherman L A. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- 49.Overwijk W W, Lee D S, Surman D R, Irvine K R, Touloukian C E, Chan C C, Carroll M W, Moss B, Rosenberg S A, Restifo N P. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu J, Kindsvogel W, Busby S, Bailey M C, Shi Y Y, Greenberg P D. J Exp Med. 1993;177:1681–1690. doi: 10.1084/jem.177.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uyttenhove C, Godfraind C, Lethae B, Amar-Costesec A, Renauld J C, Gajewski T F, Duffour M T, Warnier G, Boon T, Van den Eynde B J. Int J Cancer. 1997;70:349–356. doi: 10.1002/(sici)1097-0215(19970127)70:3<349::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]