Abstract
Xylan-degrading enzymes were induced when Phanerochaete chrysosporium was grown at 30°C in shake flask media containing xylan, Avicel PH 102, or ground corn stalks. The highest xylanase activity was produced in the corn stalk medium, while the xylan-based fermentation resulted in the lowest induction. Analytical and preparative isoelectric focusing were used to characterize xylanase multienzyme components. Preparative focusing was performed only with the cultures grown on Avicel and corn stalk. Of over 30 protein bands separated by analytical focusing from the Avicel and corn stalk media, three main groups (I, II, and III) of about five isoenzymes each showed xylanase activity when a zymogram technique with a xylan overlay was used. Enzyme assays revealed the presence of 1,4-β-endoxylanase and arabinofuranosidase activities in all three isoenzyme groups separated by preparative isoelectric focusing. β-Xylosidase activity appeared in the first peak and also as an independent peak between peaks II and III. Denatured molecular masses for the three isoenzyme groups were found to be between 18 and 90 kDa, and pI values were in the range of 4.2 to 6.0. β-Xylosidase has an apparent molecular mass of 20, 30, and 90 kDa (peak I) and 18 and 45 kDa (independent peak), indicating a trimer and dimer structure, respectively, with pI values of 4.2 and 5.78, respectively. Three more minor xylanase groups were produced on corn stalk medium: a double peak in the acidic range (pI 6.25 to 6.65 and 6.65 to 7.12) and two minor peaks in the alkaline range (pI 8.09 to 8.29 and 9.28 to 9.48, respectively). The profile of xylanases separated by isoelectric focusing (zymogram) of culture filtrate from cells grown on corn stalk media was more complex than that of culture supernatants from cells grown on cellulose. The pH optima of the three major xylanase groups are in the range of pH 4 to 5.5.
Full text
PDF

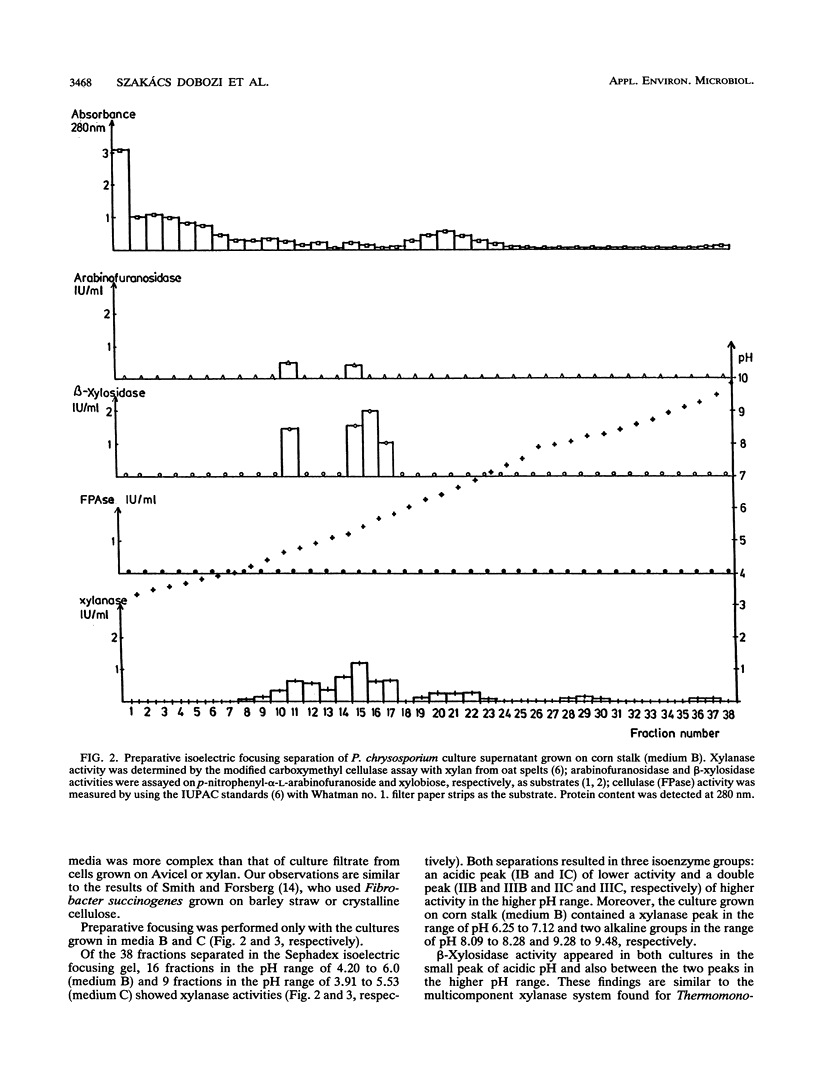
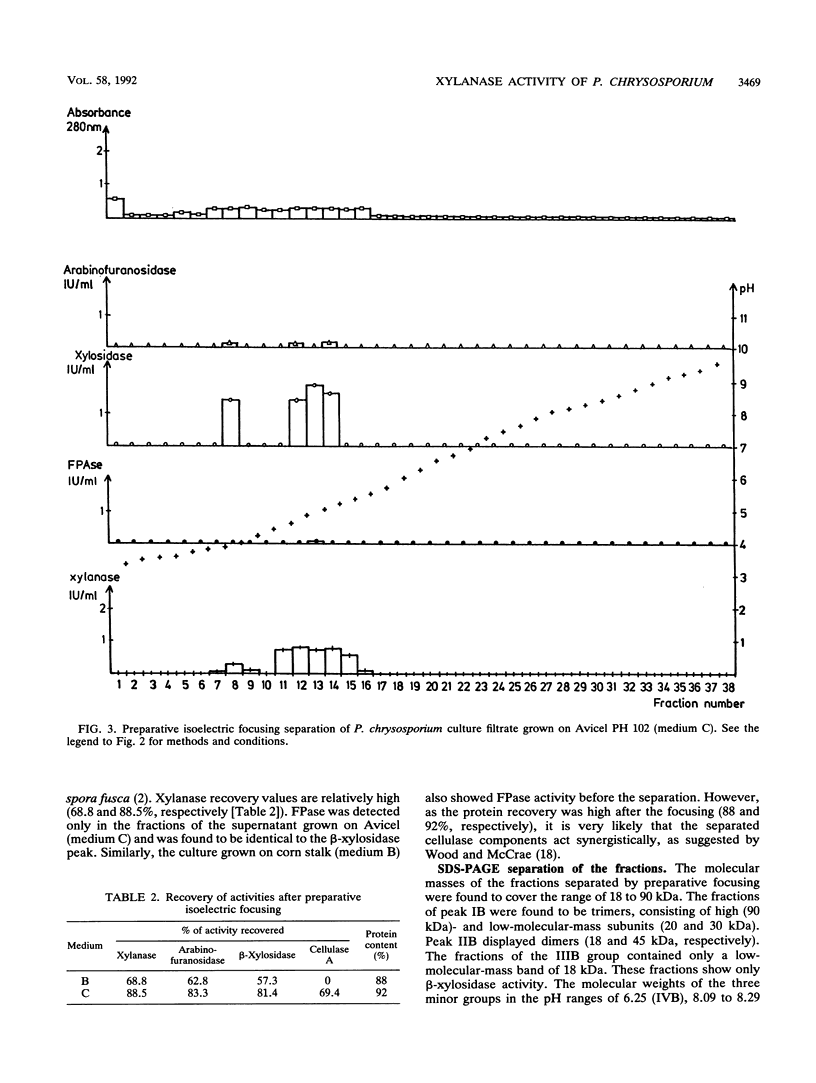


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann S. L., McCarthy A. J. Purification and Cooperative Activity of Enzymes Constituting the Xylan-Degrading System of Thermomonospora fusca. Appl Environ Microbiol. 1991 Aug;57(8):2121–2130. doi: 10.1128/aem.57.8.2121-2130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Bettermann A., Kirk T. K. Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporium during wood decay. Appl Environ Microbiol. 1991 May;57(5):1453–1460. doi: 10.1128/aem.57.5.1453-1460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 1. Separation, purification and physico-chemical characterization of five endo-1,4-beta-glucanases. Eur J Biochem. 1975 Feb 3;51(1):193–206. doi: 10.1111/j.1432-1033.1975.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Extracellular enzyme system utilized by the fungus Sporotrichum pulverulentum (Chrysosporium lignorum) for the breakdown of cellulose. 3. Purification and physico-chemical characterization of an exo-1,4-beta-glucanase. Eur J Biochem. 1975 Feb 3;51(1):213–218. doi: 10.1111/j.1432-1033.1975.tb03921.x. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Royer J. C., Nakas J. P. Simple, sensitive zymogram technique for detection of xylanase activity in polyacrylamide gels. Appl Environ Microbiol. 1990 Jun;56(6):1516–1517. doi: 10.1128/aem.56.6.1516-1517.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. C., Forsberg C. W. alpha-Glucuronidase and Other Hemicellulase Activities of Fibrobacter succinogenes S85 Grown on Crystalline Cellulose or Ball-Milled Barley Straw. Appl Environ Microbiol. 1991 Dec;57(12):3552–3557. doi: 10.1128/aem.57.12.3552-3557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Ujiie M., Roy C., Yaguchi M. Low-molecular-weight xylanase from Trichoderma viride. Appl Environ Microbiol. 1991 Jun;57(6):1860–1862. doi: 10.1128/aem.57.6.1860-1862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzcategui E., Ruiz A., Montesino R., Johansson G., Pettersson G. The 1,4-beta-D-glucan cellobiohydrolases from Phanerochaete chrysosporium. I. A system of synergistically acting enzymes homologous to Trichoderma reesei. J Biotechnol. 1991 Jul;19(2-3):271–285. doi: 10.1016/0168-1656(91)90064-3. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]



