Abstract
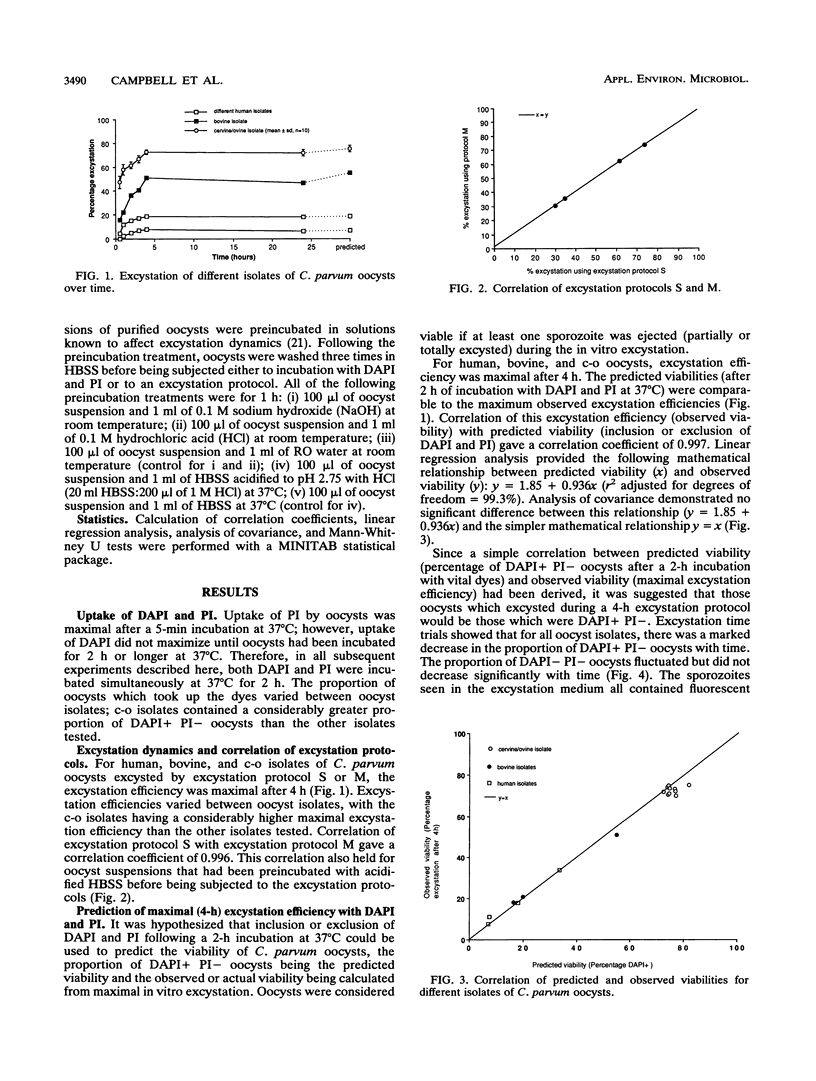
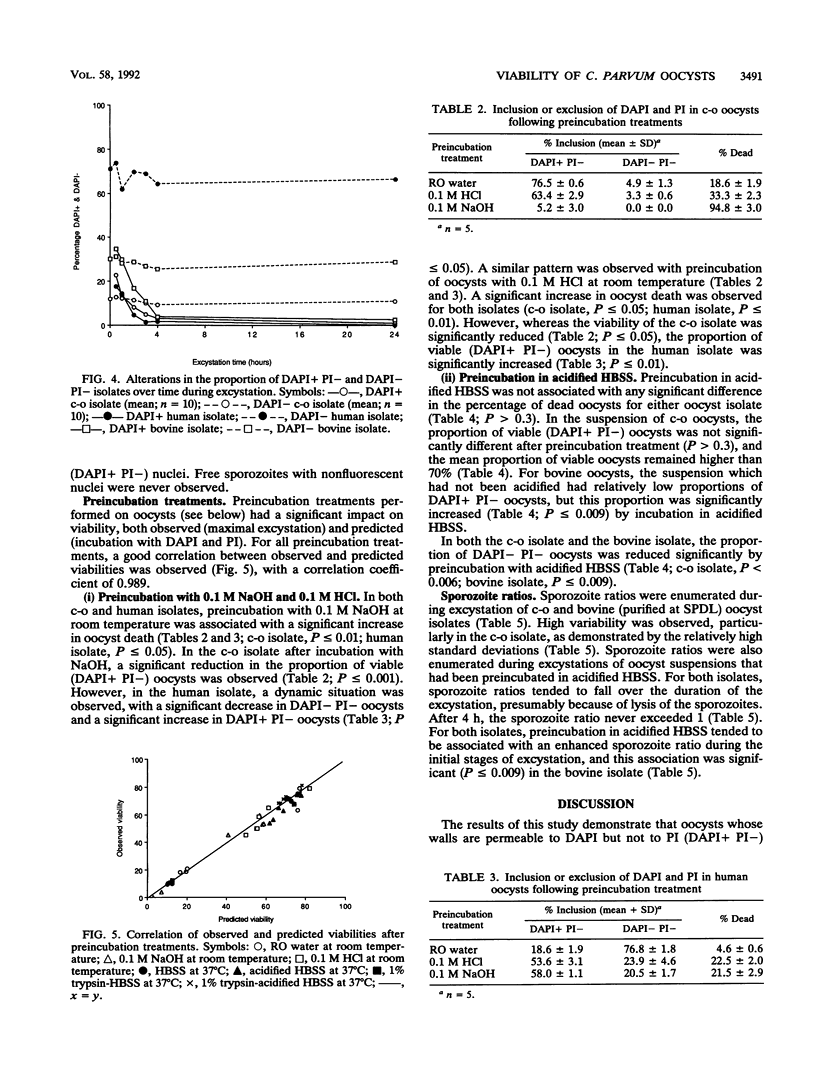
A viability assay for oocysts of Cryptosporidium parvum based on the inclusion or exclusion of two fluorogenic vital dyes, 4',6-diamidino-2-phenylindole (DAPI) and propidium iodide, was developed by using several different isolates of oocysts. Correlation of this assay with viability measured by in vitro excystation was highly statistically significant, with a calculated correlation coefficient of 0.997. In this research, two similar excystation protocols were utilized, and no significant difference between excystation protocols was detected. Percent excystation of oocyst suspensions could be increased or reduced by inclusion of a preincubation treatment in either excystation protocol, and this alteration was also demonstrated in the viability assay. Oocysts which excluded both dyes would not excyst in vitro unless a further trigger was provided and were more resistant to acid or alkali treatment. The results of this research provide a reproducible, user-friendly assay which is applicable to individual oocysts and also provides a useful adjunct for identification of oocysts in water and environmental samples.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham A. K., Jarroll E. L., Jr, Meyer E. A., Radulescu S. Giardia sp.: physical factors of excystation in vitro, and excystation vs eosin exclusion as determinants of viability. Exp Parasitol. 1979 Apr;47(2):284–291. doi: 10.1016/0014-4894(79)90080-8. [DOI] [PubMed] [Google Scholar]
- Bingham A. K., Meyer E. A. Giardia excystation can be induced in vitro in acidic solutions. Nature. 1979 Jan 25;277(5694):301–302. doi: 10.1038/277301a0. [DOI] [PubMed] [Google Scholar]
- Campbell I., Tzipori A. S., Hutchison G., Angus K. W. Effect of disinfectants on survival of cryptosporidium oocysts. Vet Rec. 1982 Oct 30;111(18):414–415. doi: 10.1136/vr.111.18.414. [DOI] [PubMed] [Google Scholar]
- Fayer R., Leek R. G. The effects of reducing conditions, medium, pH, temperature, and time on in vitro excystation of Cryptosporidium. J Protozool. 1984 Nov;31(4):567–569. doi: 10.1111/j.1550-7408.1984.tb05504.x. [DOI] [PubMed] [Google Scholar]
- Horan P. K., Kappler J. W. Automated fluorescent analysis for cytotoxicity assays. J Immunol Methods. 1977;18(3-4):309–316. doi: 10.1016/0022-1759(77)90184-3. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Kasprzak W., Majewska A. C. Infectivity of Giardia sp. cysts in relation to eosin exclusion and excystation in vitro. Tropenmed Parasitol. 1983 Mar;34(1):70–72. [PubMed] [Google Scholar]
- Korich D. G., Mead J. R., Madore M. S., Sinclair N. A., Sterling C. R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol. 1990 May;56(5):1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubista M., Akerman B., Nordén B. Characterization of interaction between DNA and 4',6-diamidino-2-phenylindole by optical spectroscopy. Biochemistry. 1987 Jul 14;26(14):4545–4553. doi: 10.1021/bi00388a057. [DOI] [PubMed] [Google Scholar]
- Labatiuk C. W., Schaefer F. W., 3rd, Finch G. R., Belosevic M. Comparison of animal infectivity, excystation, and fluorogenic dye as measures of Giardia muris cyst inactivation by ozone. Appl Environ Microbiol. 1991 Nov;57(11):3187–3192. doi: 10.1128/aem.57.11.3187-3192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters J. E., Mazás E. A., Masschelein W. J., Villacorta Martiez de Maturana I., Debacker E. Effect of disinfection of drinking water with ozone or chlorine dioxide on survival of Cryptosporidium parvum oocysts. Appl Environ Microbiol. 1989 Jun;55(6):1519–1522. doi: 10.1128/aem.55.6.1519-1522.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice E. W., Schaefer F. W., 3rd Improved in vitro excystation procedure for Giardia lamblia cysts. J Clin Microbiol. 1981 Dec;14(6):709–710. doi: 10.1128/jcm.14.6.709-710.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Stevens D. P., Mahmoud A. A., Warren K. S. Giardiasis in the mouse: an animal model. Gastroenterology. 1976 Jul;71(1):57–61. [PubMed] [Google Scholar]
- Robertson L. J., Campbell A. T., Smith H. V. Survival of Cryptosporidium parvum oocysts under various environmental pressures. Appl Environ Microbiol. 1992 Nov;58(11):3494–3500. doi: 10.1128/aem.58.11.3494-3500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp D. G., Erlandsen S. L. A new method to determine Giardia cyst viability: correlation of fluorescein diacetate and propidium iodide staining with animal infectivity. Appl Environ Microbiol. 1987 Apr;53(4):704–707. doi: 10.1128/aem.53.4.704-707.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Smith H. V. A comparison of fluorescein diacetate and propidium iodide staining and in vitro excystation for determining Giardia intestinalis cyst viability. Parasitology. 1989 Dec;99(Pt 3):329–331. doi: 10.1017/s0031182000059035. [DOI] [PubMed] [Google Scholar]
- Smith H. V., Rose J. B. Waterborne cryptosporidiosis. Parasitol Today. 1990 Jan;6(1):8–12. doi: 10.1016/0169-4758(90)90378-h. [DOI] [PubMed] [Google Scholar]



