Abstract
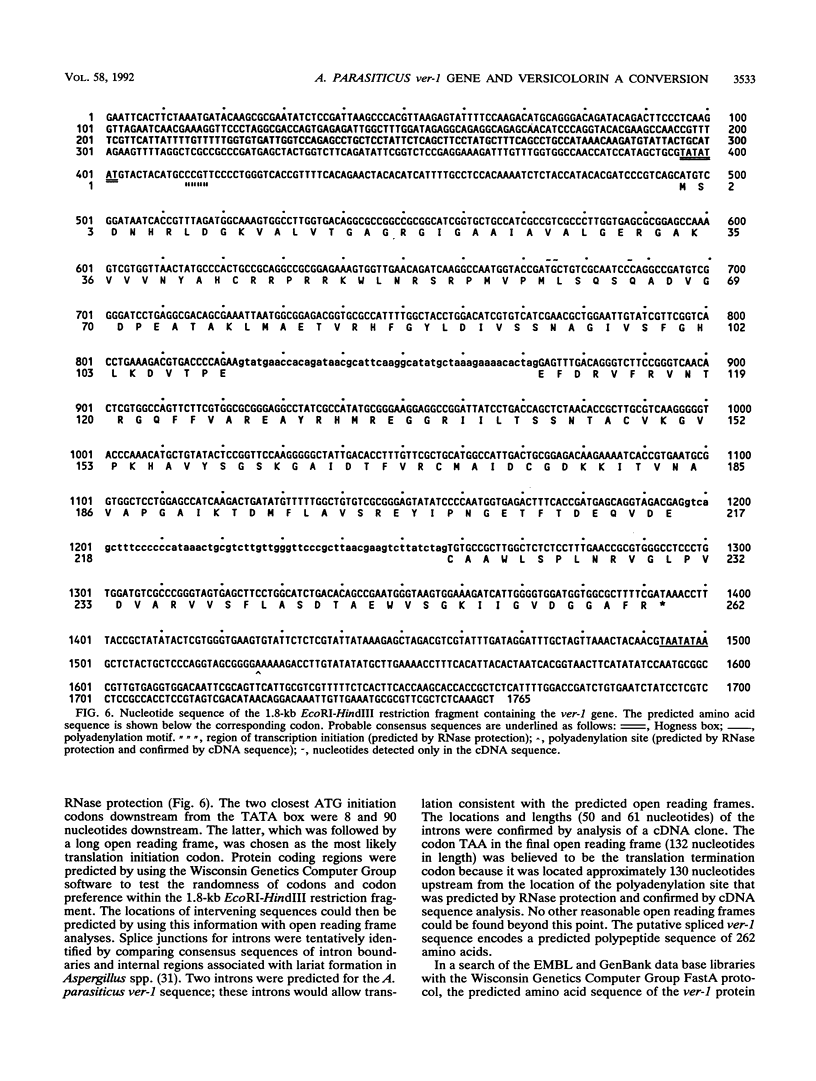
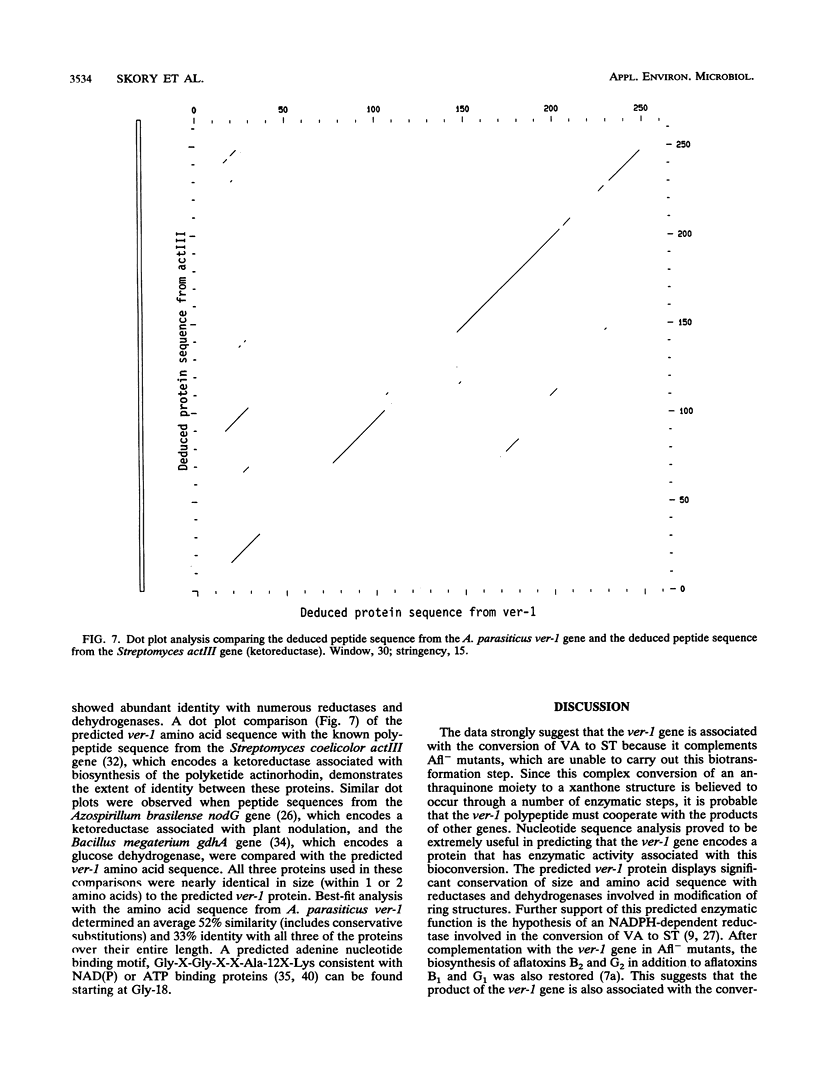
DNA isolated from the wild-type aflatoxin-producing (Afl+) fungus Aspergillus parasiticus NRRL 5862 was used to construct a cosmid genomic DNA library employing the homologous gene (pyrG) encoding orotidine monophosphate decarboxylase for selection of fungal transformants. The cosmid library was transformed into an Afl- mutant, A. parasiticus CS10 (ver-1 wh-1 pyrG), deficient in the conversion of the aflatoxin biosynthetic intermediate versicolorin A to sterigmatocystin. One pyrG+ Afl+ transformant was identified. DNA fragments from this transformant, recovered by marker rescue, contained part of the cosmid vector including the pyrG gene, the ampr gene, and a piece of the original genomic insert DNA. Transformation of these rescued DNA fragments into A. parasiticus CS10 resulted in production of wild-type levels of aflatoxin and abundant formation of sclerotia. The gene responsible for this complementation (ver-1) was identified by Northern RNA analysis and transformation with subcloned DNA fragments. The approximate locations of transcription initiation and polyadenylation sites of ver-1 were determined by an RNase protection assay and cDNA sequence analysis. The predicted amino acid sequence, deduced from the ver-1 genomic and cDNA nucleotide sequences, was compared with the EMBL and GenBank data bases. The search revealed striking similarity with Streptomyces ketoreductases involved in polyketide biosynthesis.
Full text
PDF
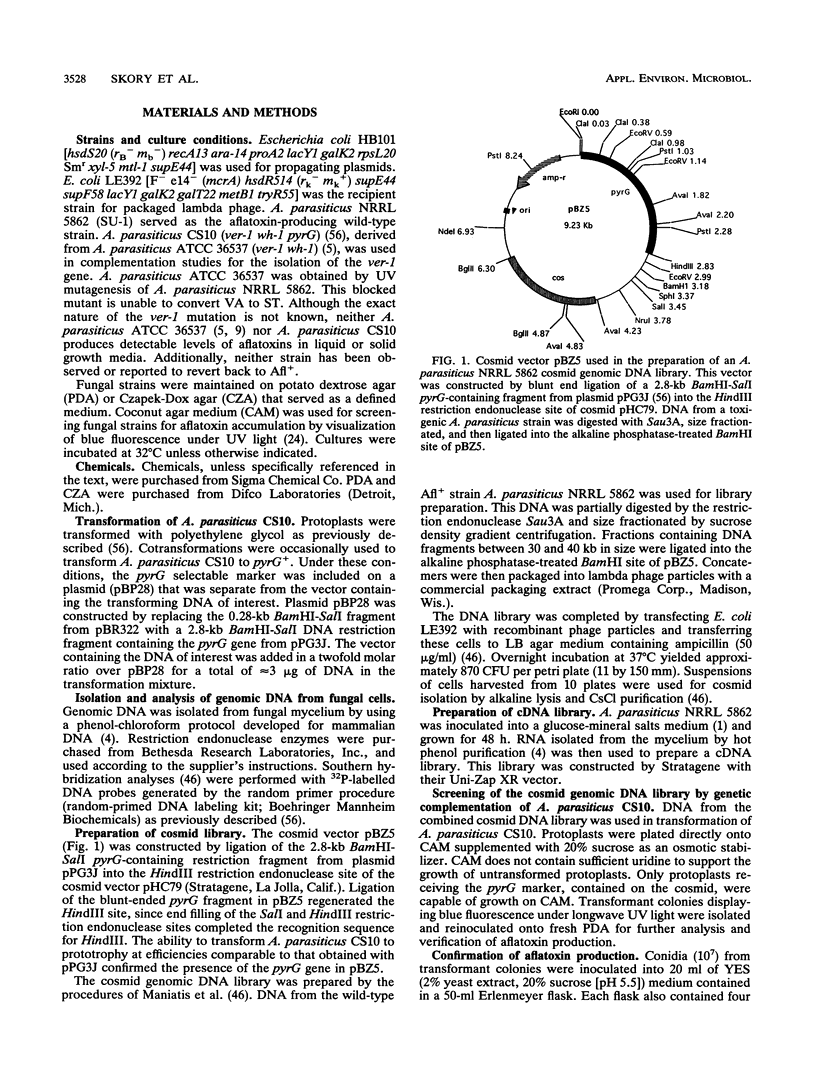
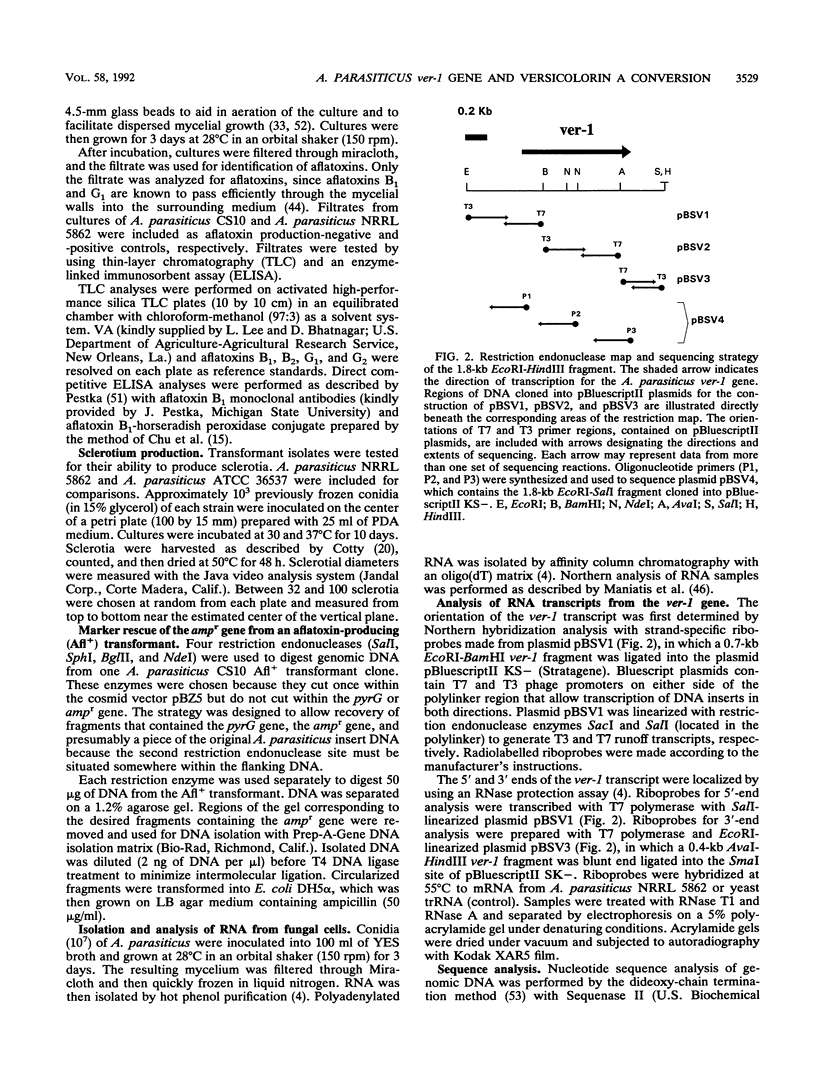





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADYE J., MATELES R. I. INCORPORATION OF LABELLED COMPOUNDS INTO AFLATOXINS. Biochim Biophys Acta. 1964 May 11;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Chung C. H., Cho S. H. Versicolorin A hemiacetal, hydroxydihydrosterigmatocystin, and aflatoxin G2 alpha reductase activity in extracts from Aspergillus parasiticus. Mycopathologia. 1990 Jul;111(1):39–45. doi: 10.1007/BF02277300. [DOI] [PubMed] [Google Scholar]
- Anderson J. A., Chung C. H. Conversion of versiconal acetate to versiconal and versicolorin C in extracts from Aspergillus parasiticus. Mycopathologia. 1990 Apr;110(1):31–35. doi: 10.1007/BF00442767. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Goldblatt L. A. The isolation of mutants of Aspergillus flavus and A.parasiticus with altered aflatoxin producing ability. Sabouraudia. 1973 Nov;11(3):235–241. [PubMed] [Google Scholar]
- Bhatnagar D., Ullah A. H., Cleveland T. E. Purification and characterization of a methyltransferase from Aspergillus parasiticus SRRC 163 involved in aflatoxin biosynthetic pathway. Prep Biochem. 1988;18(3):321–349. doi: 10.1080/00327488808062532. [DOI] [PubMed] [Google Scholar]
- Caballero J. L., Martinez E., Malpartida F., Hopwood D. A. Organisation and functions of the actVA region of the actinorhodin biosynthetic gene cluster of Streptomyces coelicolor. Mol Gen Genet. 1991 Dec;230(3):401–412. doi: 10.1007/BF00280297. [DOI] [PubMed] [Google Scholar]
- Chang P. K., Skory C. D., Linz J. E. Cloning of a gene associated with aflatoxin B1 biosynthesis in Aspergillus parasiticus. Curr Genet. 1992 Mar;21(3):231–233. doi: 10.1007/BF00336846. [DOI] [PubMed] [Google Scholar]
- Chu F. S., Hsia M. T., Sun P. S. Preparation and characterization of aflatox-n B1-1-(O-carboxymethyl) oxime. J Assoc Off Anal Chem. 1977 Jul;60(4):791–794. [PubMed] [Google Scholar]
- Chu F. S. Mycotoxins: food contamination, mechanism, carcinogenic potential and preventive measures. Mutat Res. 1991 Mar-Apr;259(3-4):291–306. doi: 10.1016/0165-1218(91)90124-5. [DOI] [PubMed] [Google Scholar]
- Chuturgoon A. A., Dutton M. F., Berry R. K. The preparation of an enzyme associated with aflatoxin biosynthesis by affinity chromatography. Biochem Biophys Res Commun. 1990 Jan 15;166(1):38–42. doi: 10.1016/0006-291x(90)91908-b. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E. Conversion of dihydro-O-methylsterigmatocystin to aflatoxin B2 by Aspergillus parasiticus. Arch Environ Contam Toxicol. 1989 May-Jun;18(3):429–433. doi: 10.1007/BF01062369. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Lax A. R., Lee L. S., Bhatnagar D. Appearance of enzyme activities catalyzing conversion of sterigmatocystin to aflatoxin B1 in late-growth-phase Aspergillus parasiticus cultures. Appl Environ Microbiol. 1987 Jul;53(7):1711–1713. doi: 10.1128/aem.53.7.1711-1713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins R. J. Indirect costs: FASEB looks for the answers. FASEB J. 1991 Sep;5(12):2623–2623. doi: 10.1096/fasebj.5.12.1916085. [DOI] [PubMed] [Google Scholar]
- Davis N. D., Iyer S. K., Diener U. L. Improved method of screening for aflatoxin with a coconut agar medium. Appl Environ Microbiol. 1987 Jul;53(7):1593–1595. doi: 10.1128/aem.53.7.1593-1595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. K., Chater K. F. Spore colour in Streptomyces coelicolor A3(2) involves the developmentally regulated synthesis of a compound biosynthetically related to polyketide antibiotics. Mol Microbiol. 1990 Oct;4(10):1679–1691. doi: 10.1111/j.1365-2958.1990.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Delledonne M., Porcari R., Fogher C. Nucleotide sequence of the nodG gene of Azospirillum brasilense. Nucleic Acids Res. 1990 Nov 11;18(21):6435–6435. doi: 10.1093/nar/18.21.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K. Effect on aflatoxin production of competition between wild-type and mutant strains of Aspergillus parasiticus. Mycopathologia. 1987 Feb;97(2):93–96. doi: 10.1007/BF00436844. [DOI] [PubMed] [Google Scholar]
- Feng G. H., Chu F. S., Leonard T. J. Molecular cloning of genes related to aflatoxin biosynthesis by differential screening. Appl Environ Microbiol. 1992 Feb;58(2):455–460. doi: 10.1128/aem.58.2.455-460.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T. J., Coulson A. R., Sulston J. E., Little P. F. Lorist2, a cosmid with transcriptional terminators insulating vector genes from interference by promoters within the insert: effect on DNA yield and cloned insert frequency. Gene. 1987;53(2-3):275–281. doi: 10.1016/0378-1119(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Hallam S. E., Malpartida F., Hopwood D. A. Nucleotide sequence, transcription and deduced function of a gene involved in polyketide antibiotic synthesis in Streptomyces coelicolor. Gene. 1988 Dec 30;74(2):305–320. doi: 10.1016/0378-1119(88)90165-5. [DOI] [PubMed] [Google Scholar]
- Heilmann H. J., Mägert H. J., Gassen H. G. Identification and isolation of glucose dehydrogenase genes of Bacillus megaterium M1286 and their expression in Escherichia coli. Eur J Biochem. 1988 Jun 15;174(3):485–490. doi: 10.1111/j.1432-1033.1988.tb14124.x. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Suzuki H., Beppu T. Nucleotide sequence of afsB, a pleiotropic gene involved in secondary metabolism in Streptomyces coelicolor A3(2) and "Streptomyces lividans". J Bacteriol. 1986 Oct;168(1):257–269. doi: 10.1128/jb.168.1.257-269.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng J. S., Linz J. E., Pestka J. J. Cloning and characterization of the trpC gene from an aflatoxigenic strain of Aspergillus parasiticus. Appl Environ Microbiol. 1989 Oct;55(10):2561–2568. doi: 10.1128/aem.55.10.2561-2568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh D. P., Wan C. C., Billington J. A. A versiconal hemiacetal acetate converting enzyme in aflatoxin biosynthesis. Mycopathologia. 1989 Sep;107(2-3):121–126. doi: 10.1007/BF00707548. [DOI] [PubMed] [Google Scholar]
- Jelinek C. F., Pohland A. E., Wood G. E. Worldwide occurrence of mycotoxins in foods and feeds--an update. J Assoc Off Anal Chem. 1989 Mar-Apr;72(2):223–230. [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Bennett J. W., Cucullu A. F., Stanley J. B. Synthesis of versicolorin A by a mutant strain of Aspergillus parasiticus deficient in aflatoxin production. J Agric Food Chem. 1975 Nov-Dec;23(6):1132–1134. doi: 10.1021/jf60202a011. [DOI] [PubMed] [Google Scholar]
- Lin B. K., Anderson J. A. Purification and properties of versiconal cyclase from Aspergillus parasiticus. Arch Biochem Biophys. 1992 Feb 14;293(1):67–70. doi: 10.1016/0003-9861(92)90366-5. [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Ayres J. C., Koehler P. E. Influence of temperature cycling on the production of aflatoxins B1 and G1 by Aspergillus parasiticus. Appl Environ Microbiol. 1980 Aug;40(2):333–336. doi: 10.1128/aem.40.2.333-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. T., Sharp P. M. Codon usage in Aspergillus nidulans. Mol Gen Genet. 1991 Nov;230(1-2):288–294. doi: 10.1007/BF00290679. [DOI] [PubMed] [Google Scholar]
- Papa K. E. Genetics of Aspergillus flavus: linkage of aflatoxin mutants. Can J Microbiol. 1984 Jan;30(1):68–73. doi: 10.1139/m84-012. [DOI] [PubMed] [Google Scholar]
- Pestka J. J. Enhanced surveillance of foodborne mycotoxins by immunochemical assay. J Assoc Off Anal Chem. 1988 Nov-Dec;71(6):1075–1081. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. H., Malpartida F., Bibb M. J., Kieser H. M., Bibb M. J., Hopwood D. A. Structure and deduced function of the granaticin-producing polyketide synthase gene cluster of Streptomyces violaceoruber Tü22. EMBO J. 1989 Sep;8(9):2717–2725. doi: 10.1002/j.1460-2075.1989.tb08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Horng J. S., Pestka J. J., Linz J. E. Transformation of Aspergillus parasiticus with a homologous gene (pyrG) involved in pyrimidine biosynthesis. Appl Environ Microbiol. 1990 Nov;56(11):3315–3320. doi: 10.1128/aem.56.11.3315-3320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W. E. Molecular genetics of Aspergillus development. Annu Rev Genet. 1990;24:5–36. doi: 10.1146/annurev.ge.24.120190.000253. [DOI] [PubMed] [Google Scholar]
- Yabe K., Ando Y., Hashimoto J., Hamasaki T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl Environ Microbiol. 1989 Sep;55(9):2172–2177. doi: 10.1128/aem.55.9.2172-2177.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Nakamura Y., Nakajima H., Ando Y., Hamasaki T. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl Environ Microbiol. 1991 May;57(5):1340–1345. doi: 10.1128/aem.57.5.1340-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]