Abstract
Islet amyloid contributes to the loss of β-cell mass in type 2 diabetes. To examine the roles of glucose and time on amyloid formation, we developed a rapid in vitro model using isolated islets from human islet amyloid polypeptide (hIAPP) transgenic mice. Islets from hIAPP transgenic and non-transgenic mice were cultured for up to 7 days with either 5.5, 11.1, 16.7 or 33.3 mmol/l glucose. At various time-points throughout the culture period, islets were harvested for determination of amyloid and β-cell areas, and for measures of cell viability, insulin content and secretion. Following culture of hIAPP transgenic islets in 16.7 or 33.3 mmol/l glucose, amyloid formation was significantly increased compared to 5.5 or 11.1 mmol/l glucose culture. Amyloid was detected as early as day 2 and increased in a time-dependent manner so that by day 7, a decrease in the proportion of β-cell area in hIAPP transgenic islets was evident. When compared to non-transgenic islets after 7-day culture in 16.7 mmol/l glucose, hIAPP transgenic islets were 24% less viable, had decreased β-cell area and insulin content, but displayed no change in insulin secretion. Thus, we have developed a rapid in vitro model of light microscopy-visible islet amyloid formation that is both glucose- and time-dependent. Formation of amyloid in this model is associated with reduced cell viability and β-cell loss but adequate functional adaptation. It thus enables studies investigating the mechanism(s) underlying the amyloid-associated loss of β-cell mass in type 2 diabetes.
Keywords: islet amyloid, human islet amyloid polypeptide, islets, ß-cell, insulin secretion, cell viability, amylin, mouse model, diabetes
Islet amyloid polypeptide (IAPP) is a normal product of the pancreatic β-cell. Human, but not rodent, IAPP is capable of forming amyloid deposits, which are associated with loss of both β-cell mass and normal islet function [1, 2]. Models have been developed to elucidate the underlying mechanisms responsible for the deleterious effects of amyloidogenesis. Initially, these involved the extracellular application of human IAPP (hIAPP) to immortalized cells [3], or the transfection of cells that lack the sophisticated secretory machinery that is typical of the β-cell [4]. While cell death and/or impaired insulin secretion were demonstrated, these models do not depict what is occurring in humans.
Advancement of transgene technology has enabled a more informative approach to studying amyloidogenesis, where hIAPP could be targeted to rodent β-cells and use of various strategies could induce the deposition of islet amyloid (reviewed in [5]). In our model, we have observed that hIAPP transgenic mice develop light microscopy-visible amyloid after 12–16 months on a 9% fat diet [6]. This occurs predominantly in male mice, as female mice are protected by ovarian products [7]. The utility of our mouse model and others like it [8, 9] has been somewhat limited since studies typically take a year to perform and it is not possible to discern a direct role of a given intervention on islet amyloid formation. Furthermore, female mice are generally not useful for in vivo studies due to the low incidence of amyloid deposition. Of note, an improved system has been developed to study islet amyloid using isolated islets but this relies on labour-intensive electron microscopy to detect amyloid deposition [10–12]. For these reasons, we sought to develop a rapid model of islet amyloid formation, where amyloid can be easily visualized by light microscopy. In the present study, we have utilized an in vitro approach, namely culture of isolated islets from our male and female hIAPP transgenic mice. Our findings provide insight into the potential role of islet amyloid in the progression of type 2 diabetes.
Materials and Methods
Transgenic Mice
Hemizygous transgenic mice expressing hIAPP in their pancreatic ß-cells were generated as previously described [6] by breeding hIAPP transgenic C57BL/6 female mice with DBA/2J wild type male mice. Mice were fed a diet containing 9% fat by weight (Purina #5021). The study was approved by the Institutional Animal Care and Use Committee at the Seattle VA Puget Sound Health Care System.
Isolation of pancreatic islets
Islets were isolated from the pancreata of 10-week old male and female mice as previously described [13]. After overnight recovery, islets were transferred to culture media containing 5.5, 11.1, 16.7 or 33.3 mmol/l glucose (denoted day 1 throughout the manuscript). Islets were subsequently cultured for up to 7 days, with the media being replaced every 48 hours.
Histological assessment of islet amyloid and β-cells
At various time points throughout the culture period, 50 islets were removed from the culture plates, fixed in 4% (w/v) phosphate-buffered paraformaldehyde and embedded in paraffin. Five-μm sections were cut and co-stained with thioflavin S to visualize amyloid deposits and with anti-insulin antibody (1:2000; Sigma, St. Louis, Mo, USA) followed by Cy3-conjugated anti-mouse immunoglobulins to visualize islet β-cells. Histological assessments were made on an average of 27 islets per plate. From these measures, islet amyloid prevalence (% of islets containing amyloid), amyloid severity (% of islet area occupied by amyloid), β-cell area and islet area were determined using a computer-based quantitative method as reported previously [14]. Islet area was determined morphometrically by manually outlining each islet when viewed under fluorescence at excitation 480 nm and emission 505 nm (channel used for thioflavin S staining).
Cell Viability Assay
Islet cell viability after 7 days of 16.7 mmol/l glucose culture was assessed using the Cell Proliferation Kit I according to the manufacturer’s instructions (Roche, Indianapolis, IN, USA). This assay is based on the ability of viable cells to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to insoluble coloured formazan crystals. Briefly, islets were plated at 20/well (duplicates for each condition) and 0.5% (w/v) MTT was added to 100 μL of culture medium in each well. After 4 h at 37°C, 100 μL solubilization solution was added. After overnight incubation, solubilized formazan was quantified spectrophotometrically.
Perifusion of islets for insulin secretion
After 7 days of 16.7 mmol/l glucose culture, 100 islets were collected and loaded into a perifusion chamber. Islets were first perifused with Krebs-Ringer bicarbonate buffer (KRB; 98.5 mmol/l NaCl, 5.5 mmol/l NaHCO3, 4.9 mmol/l KCl, 1.2 mmol/l KH2PO4, 1.2 mmol/l MgSO4, 2.6 mmol/l CaCl2, 20 mmol/l HEPES and 0.1% BSA, pH 7.4) containing 1.67 mmol/l glucose for 1 h to achieve basal insulin secretion. Effluent fractions were then collected at 2–5 minute intervals during perifusion with 1.67 mmol/l glucose for 8 minutes, then with 16.7 mmol/l glucose for 30 minutes, followed by 16.7 mmol/l glucose with 10 mmol/l arginine and 0.01 mmol/l IBMX for 30 minutes. Finally, islets were perifused with 1.67 mmol/l glucose for 20 minutes. Samples were stored at −20°C before determination of insulin by radioimmunoassay. Islet insulin content was also measured after acid-ethanol extraction.
Statistical Analyses
Data are mean ± SEM for the number of experiments indicated. Mean data for glucose dose-response studies and comparison of hIAPP transgenic versus non-transgenic islets were performed using analysis of variance or t-tests respectively, with non-parametric tests being used where data were not normally distributed. For time course data, a repeated measures analysis of variance was used. A p<0.05 was considered statistically significant.
Results
Effect of gender on islet amyloid formation in vitro
To determine whether gender affects islet amyloid formation in vitro, islets from male and female hIAPP transgenic mice were cultured for 7 days in 16.7 mmol/l glucose. Following thioflavin S staining, amyloid prevalence (% of islets containing amyloid) and severity (% of islet area occupied by amyloid) were calculated. Neither amyloid prevalence (80 ± 11 vs 52 ± 15%; n=4, p=0.19) nor severity (0.79 ± 0.34 vs 0.75 ± 0.49%; n=4, p=0.96) differed between islets from male versus female mice. Therefore all subsequent studies were performed using a mix of male and female islets.
Effect of glucose concentration on islet amyloid formation and β-cell area
Islets from hIAPP transgenic mice were isolated and cultured for 7 days in 5.5, 11.1, 16.7 or 33.3 mmol/l glucose, at which time amyloid prevalence and severity were determined. As shown in Figure 1a, 36% and 57% of the islets cultured in 16.7 mmol/l and 33.3 mmol/l glucose respectively, contained light microscopy-visible amyloid, compared with only 7% and 6% of the islets cultured in either 5.5 mmol/l or 11.1 mmol/l glucose respectively. Similarly, amyloid severity (Figure 1b) was significantly increased after 7-day culture in either 16.7 mmol/l or 33.3 mmol/l glucose, compared with 5.5 mmol/l and 11.1 mmol/l glucose. In addition, islets cultured in 33.3 mmol/l glucose exhibited significantly greater islet amyloid prevalence and severity than islets cultured in 16.7 mmol/l glucose.
Figure 1.
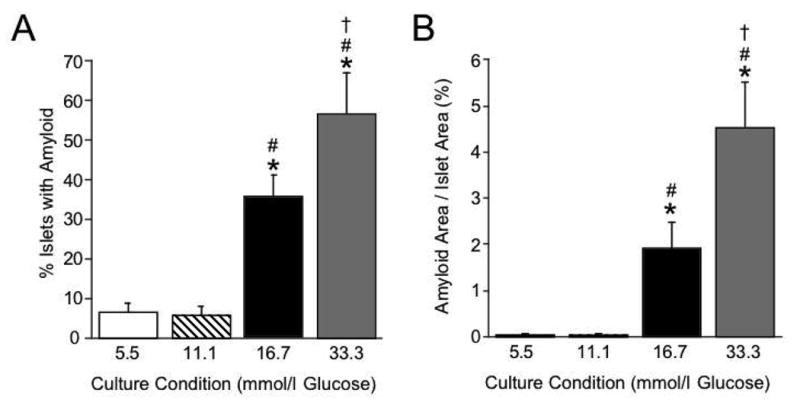
Amyloid prevalence (% islets containing amyloid; panel A) and severity (% islet area occupied by amyloid; panel B) in hIAPP transgenic mouse islets cultured for 7 days in 5.5 (open bars; n=6), 11.1 (hatched bars; n=6), 16.7 (black bars; n=11) or 33.3 mmol/l glucose (grey bars; n=8). Thioflavin S staining of islets at day 7 revealed a glucose-dependent effect to increase both amyloid prevalence and severity. *p<0.05 vs 5.5 mmol/l glucose; #p<0.05 vs 11.1 mmol/l glucose; †p<0.05 vs 16.7 mmol/l glucose.
To determine whether the presence of amyloid impacted β-cell area, islet sections were immunolabelled for insulin after which insulin-positive area was quantified as a proportion of islet area. There was no significant difference in β-cell area between islets cultured for 7 days in 5.5 mmol/l versus 11.1 mmol/l glucose (57.4 ± 3.8% vs 64.8 ± 2.3%; n=6, p=0.09). However, after 7-day culture in 16.7 mmol/l glucose, β-cell area was significantly decreased to 47.5 ± 1.9% (n=11; p<0.01 vs 5.5 mmol/l and 11.1 mmol/l glucose). Similarly, 33.3 mmol/l glucose culture decreased β-cell area to 41.5 ± 2.8% (n=8; p<0.001 vs 5.5 mmol/l and 11.1 mmol/l glucose).
Effect of exposure time on islet amyloid formation
To determine whether amyloid formation and accumulation is time-dependent, hIAPP transgenic islets were cultured in 16.7 mmol/l glucose and then harvested at 24-hour intervals from the start of the culture period, for up to 144 hours (day 7). Figure 2a shows that the proportion of islets containing amyloid did not differ over the 7 days. In contrast, amyloid severity (Figure 2b) increased in a time-dependent manner so that by day 7, amyloid severity was ~5-fold greater than at 24 hours (day 2).
Figure 2.
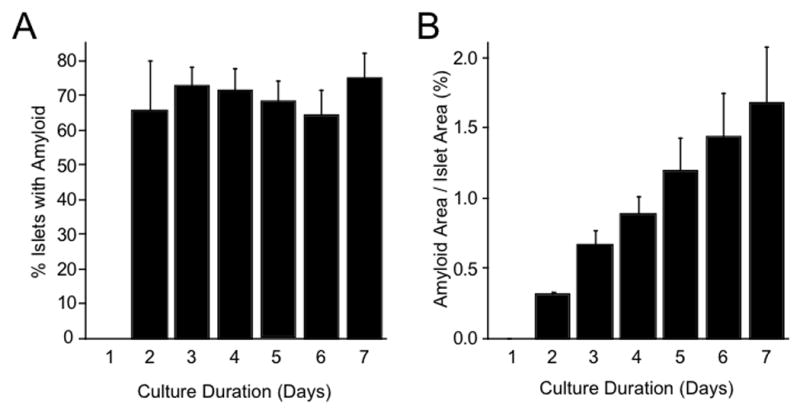
Time-dependent changes in amyloid prevalence (panel A; p=0.003 vs day 1) and severity (panel B; p=0.005 vs day 1) in hIAPP transgenic islets cultured in 16.7 mmol/l glucose and harvested every 24 hours, up to day 7. Each time point represents five separate experiments. Thioflavin S staining of islets at each time-point revealed a time-dependent increase in amyloid severity, but not prevalence.
Effect of islet amyloid on insulin secretion and insulin content
To determine whether the presence of amyloid affects β-cell function, insulin secretion was assessed by perifusing hIAPP transgenic and non-transgenic islets after 7 days of culture in 16.7 mmol/l glucose. The 16.7 mmol/l glucose culture condition was chosen since this concentration is not supraphysiological, particularly for an in vitro environment, and so the well-documented deleterious effects (other than amyloid formation) that are commonly associated with high glucose exposure could be largely avoided. Islets were perifused with 1.67 mmol/l glucose, followed successively by 16.7 mmol/l glucose and 16.7 mmol/l glucose in the presence of arginine and IBMX (to estimate maximal secretory capacity). As shown in Figure 3a, hIAPP transgenic islets secreted similar amounts of insulin in response to 16.7 mmol/l glucose both in the absence and presence of arginine and IBMX compared to non-transgenic islets, despite having significantly lower islet insulin content (Figure 3b).
Figure 3.
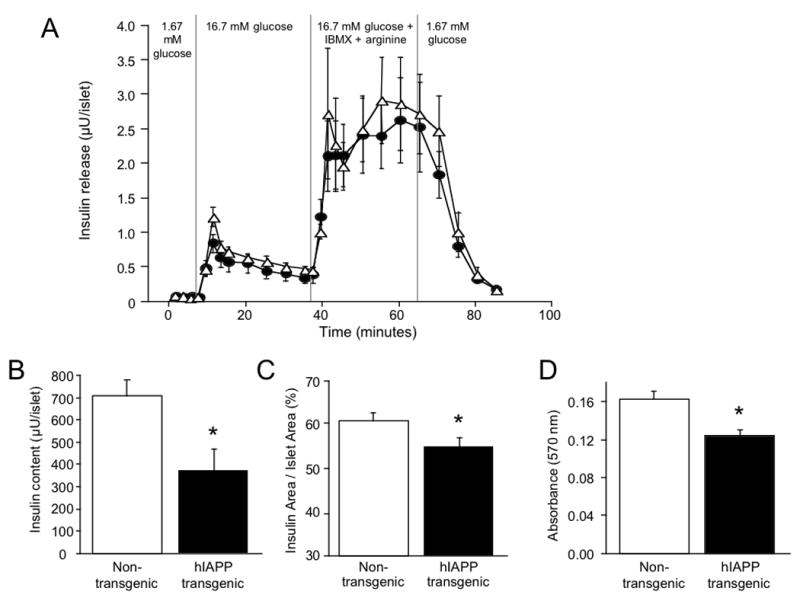
Effect of islet amyloid on insulin secretion (panel A; n=4). After 7-day culture in 16.7 mmol/l glucose, insulin secretion in response to 1.67 mmol/l glucose, 16.7 mmol/l glucose alone and in the presence of IBMX and arginine was not different between hIAPP transgenic (closed circles) and non-transgenic mouse islets (open triangles; panel A). In contrast, hIAPP transgenic islets (closed bars) had significantly reduced insulin content (panel B; n=4), β-cell area (panel C; n=8–9) and viability (panel D; n=6), compared to non-transgenic islets (open bars). *p<0.05 vs non-transgenic islets.
Effect of islet amyloid on β-cell area
To determine whether islet amyloid formation, rather than culture in 16.7 mmol/l glucose per se was associated with β-cell loss, we quantified β-cell area as a proportion of islet area in hIAPP transgenic and non-transgenic islets following 7 days of culture in 16.7 mmol/l glucose. Again, we chose this glucose condition to minimise any deleterious effects of high glucose per se. Figure 3c shows that β-cell area was significantly reduced in hIAPP transgenic islets compared to non-transgenic islets.
Effect of islet amyloid on cell viability
To determine whether the presence of amyloid affects cell viability, the MTT assay was performed after hIAPP transgenic and non-transgenic islets were cultured for 7 days in 16.7 mmol/l glucose. As shown in Figure 3d, cell viability was significantly decreased by 24% in hIAPP transgenic islets compared to non-transgenic islets.
Discussion
In this study, we describe the development of an in vitro model of islet amyloid in which amyloid deposition occurs rapidly and in which amyloid can easily be visualized by thioflavin S staining and light microscopy. Glucose concentration and time of exposure to elevated glucose were found to be important determinants of the extent of amyloid deposition. However, in contrast to previous in vivo studies using our hIAPP transgenic mouse model [7], we observed that when cultured in vitro, islets from female mice are no longer protected against amyloid deposition. Islet amyloid deposition in this in vitro model is associated with β-cell loss, decreased insulin content and decreased islet cell viability. Paradoxically, there is also an adaptive response to maintain insulin secretion after the induction of amyloid.
Various models have been created to study mechanisms for the loss of β-cell mass and function that is associated with islet amyloidogenesis [5]. Of the in vivo models, many do not develop islet amyloid unless the hIAPP gene dosage is increased by breeding to homozygosity [15], or the hIAPP expression rate is accelerated by various strategies like either cross-breeding onto an obese background [8, 9] or high fat feeding [16]. While advantageous due to the whole-body approach, these models can be costly and time-consuming, making an in vitro approach attractive. Of the in vitro models that have been used to study amyloidogenesis, many do not represent what is occurring in human disease. For instance, the treatment of islets or β-cell lines with exogenous hIAPP [17, 18] limits the understanding of how functional derangements within the cell contribute to initiation of fibril formation. Others have developed in vitro models of islet amyloid formation using isolated islets from hIAPP transgenic mice [10–12, 19, 20]. However, these studies required labour-intensive electron microscopy for quantification of amyloid deposition and cell damage, thus severely limiting the utility of these models.
Our in vitro model is particularly relevant to islet amyloid formation in humans, since the amyloid deposits in our mouse islets are histologically comparable to the classical light microscopy-visible deposits observed in human type 2 diabetes [21], and the expression of hIAPP by itself is not sufficient to induce amyloid formation. The fact that hIAPP transgenic islets did not develop significant amounts of amyloid when exposed to 5.5 or 11.1 mmol/l glucose supports the notion that a permissive environment like hyperglycemia that may be associated with β-cell dysfunction [11, 12] may be necessary to promote amyloidogenesis. We also observed a decrease in the proportion of β-cell area to islet area with the deposition of amyloid, as well as decreased cell viability and reduced insulin content, again features in keeping with those observed in human type 2 diabetes. Similar observations have been made in another in vitro model [11] and in human autopsy studies where decreased β-cell number was correlated with increased islet amyloid load [1, 22, 23]. Further, it was found that the degree of amyloid deposition was increased in individuals who required insulin treatment for their diabetes [2], presumably because their β-cells were not producing enough insulin. Despite these morphological abnormalities and the reduction in the quantity of β-cells in our in vitro model, insulin secretion was maintained in response to both glucose and non-glucose secretagogues, suggesting functional adaptation. This finding suggests that simple β-cell mass reduction is insufficient to produce β-cell dysfunction or that a greater degree of mass reduction is necessary. The ability of the reduced number of β-cells in this in vitro model to adapt functionally is in keeping with observations of β-cell functional adaptation to decreased mass in rodents [24] and dogs [25].
Exposure of islets to elevated glucose concentrations has been shown to cause a number of changes in the β-cell, many of which become more severe with time. This is illustrated in the present study where islet amyloid severity increased over 7 days of culture in 16.7 mmol/l glucose. In addition, at day 7, β-cell area, cell viability and insulin content were reduced in hIAPP transgenic islets compared to non-transgenic islets cultured under the same conditions, implicating amyloid rather than the glucose concentration per se as the cause. While amyloid-associated toxicity has been demonstrated in various other islet amyloid models [5, 18, 26], the time frame for these β-cell alterations differs greatly in comparison to our rapid model where amyloid can be seen as early as 24 hours after the start of the culture period. The glucose- and time-dependent increases in islet amyloid formation in the present study again are relevant to human type 2 diabetes. It is well recognized that type 2 diabetes is a progressive disorder and our findings suggest that the continued presence of hyperglycemia and long-standing disease duration in human diabetes could be permissive for continuous amyloid formation and thus may contribute to the progressive β-cell failure that characterizes the disease. Should this be the case, approaches to treatment and prevention that are associated with reduced amyloid deposition could preserve β-cell function and delay the need for additional therapy. This potential was highlighted in a recent study in which we demonstrated that treatment of our hIAPP transgenic mice with rosiglitazone or metformin for a year was associated with reduced amyloid formation and a preservation of β-cell function [27].
In summary, we have developed a novel in vitro model of islet amyloid formation utilizing isolated islets from hIAPP transgenic mice and believe it will be useful in gaining a more complete understanding of the detrimental effects associated with amyloidogenesis. Further, it should be useful in the development of novel approaches to inhibit islet amyloid deposition, a process that contributes to the reduction in ß-cells observed in humans with type 2 diabetes.
Acknowledgments
We thank R. Bhatti, M.J. Peters, J.I. Teague, R. Koltz, S.M. Wilbur and J.R. Willard for excellent technical support. This work was supported by research funding from the Department of Veterans Affairs and NIH grants DK-17047 and DK-74404. S.Z. was supported by an American Diabetes Association Mentor-based Fellowship.
Abbreviations
- IAPP
islet amyloid polypeptide
- hIAPP
human islet amyloid polypeptide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 2.Westermark P. Amyloid and polypeptide hormones: what is their relationship? Amyloid Int J Exp Clin Invest. 1994;1:47–60. [Google Scholar]
- 3.Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y, Kimura H. Amyloid peptides are toxic via a common oxidative mechanism. Proc Natl Acad Sci U S A. 1995;92:1989–1993. doi: 10.1073/pnas.92.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiddinga HJ, Eberhardt NL. Intracellular amyloidogenesis by human islet amyloid polypeptide induces apoptosis in COS-1 cells. Am J Pathol. 1999;154:1077–1088. doi: 10.1016/S0002-9440(10)65360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. Ilar J. 2006;47:225–233. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 6.Verchere CB, D'Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, Kahn SE. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn SE, Andrikopoulos S, Verchere CB, Wang F, Hull RL, Vidal J. Oophorectomy promotes islet amyloid formation in a transgenic mouse model of Type II diabetes. Diabetologia. 2000;43:1309–1312. doi: 10.1007/s001250051527. [DOI] [PubMed] [Google Scholar]
- 8.Hoppener JW, Oosterwijk C, Nieuwenhuis MG, Posthuma G, Thijssen JH, Vroom TM, Ahren B, Lips CJ. Extensive islet amyloid formation is induced by development of Type II diabetes mellitus and contributes to its progression: pathogenesis of diabetes in a mouse model. Diabetologia. 1999;42:427–434. doi: 10.1007/s001250051175. [DOI] [PubMed] [Google Scholar]
- 9.Soeller WC, Janson J, Hart SE, Parker JC, Carty MD, Stevenson RW, Kreutter DK, Butler PC. Islet amyloid-associated diabetes in obese A(vy)/a mice expressing human islet amyloid polypeptide. Diabetes. 1998;47:743–750. doi: 10.2337/diabetes.47.5.743. [DOI] [PubMed] [Google Scholar]
- 10.de Koning EJ, Morris ER, Hofhuis FM, Posthuma G, Hoppener JW, Morris JF, Capel PJ, Clark A, Verbeek JS. Intra- and extracellular amyloid fibrils are formed in cultured pancreatic islets of transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1994;91:8467–8471. doi: 10.1073/pnas.91.18.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacArthur DL, de Koning EJ, Verbeek JS, Morris JF, Clark A. Amyloid fibril formation is progressive and correlates with beta-cell secretion in transgenic mouse isolated islets. Diabetologia. 1999;42:1219–1227. doi: 10.1007/s001250051295. [DOI] [PubMed] [Google Scholar]
- 12.Henson MS, Buman BL, Jordan K, Rahrmann EP, Hardy RM, Johnson KH, O'Brien T D. An in vitro model of early islet amyloid polypeptide (IAPP) fibrillogenesis using human IAPP-transgenic mouse islets. Amyloid. 2006;13:250–259. doi: 10.1080/13506120600960734. [DOI] [PubMed] [Google Scholar]
- 13.Zraika S, Dunlop M, Proietto J, Andrikopoulos S. The hexosamine biosynthesis pathway regulates insulin secretion via protein glycosylation in mouse islets. Arch Biochem Biophys. 2002;405:275–279. doi: 10.1016/s0003-9861(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, Hull RL, Vidal J, Cnop M, Kahn SE. Islet amyloid develops diffusely throughout the pancreas before becoming severe and replacing endocrine cells. Diabetes. 2001;50:2514–2520. doi: 10.2337/diabetes.50.11.2514. [DOI] [PubMed] [Google Scholar]
- 15.Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, Butler PC. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hull RL, Andrikopoulos S, Verchere CB, Vidal J, Wang F, Cnop M, Prigeon RL, Kahn SE. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52:372–379. doi: 10.2337/diabetes.52.2.372. [DOI] [PubMed] [Google Scholar]
- 17.Janciauskiene S, Ahren B. Different sensitivity to the cytotoxic action of IAPP fibrils in two insulin-producing cell lines, HIT-T15 and RINm5F cells. Biochem Biophys Res Commun. 1998;251:888–893. doi: 10.1006/bbrc.1998.9574. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 19.Westermark G, Arora MB, Fox N, Carroll R, Chan SJ, Westermark P, Steiner DF. Amyloid formation in response to beta cell stress occurs in vitro, but not in vivo, in islets of transgenic mice expressing human islet amyloid polypeptide. Mol Med. 1995;1:542–553. [PMC free article] [PubMed] [Google Scholar]
- 20.Westermark GT, Gebre-Medhin S, Steiner DF, Westermark P. Islet amyloid development in a mouse strain lacking endogenous islet amyloid polypeptide (IAPP) but expressing human IAPP. Mol Med. 2000;6:998–1007. [PMC free article] [PubMed] [Google Scholar]
- 21.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 22.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 23.Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978;15:417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- 24.Lee HC, Bonner-Weir S, Weir GC, Leahy JL. Compensatory adaption to partial pancreatectomy in the rat. Endocrinology. 1989;124:1571–1575. doi: 10.1210/endo-124-3-1571. [DOI] [PubMed] [Google Scholar]
- 25.Ward WK, Wallum BJ, Beard JC, Taborsky GJJ, Porte D J. Reduction of glycemia potentiation: sensitive indicator of B-cell loss in partially pancreatectomized dogs. Diabetes. 1988;37:733–739. doi: 10.2337/diab.37.6.723. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Liu J, Dragunow M, Cooper GJ. Fibrillogenic amylin evokes islet beta-cell apoptosis through linked activation of a caspase cascade and JNK1. J Biol Chem. 2003;278:52810–52819. doi: 10.1074/jbc.M308244200. [DOI] [PubMed] [Google Scholar]
- 27.Hull RL, Shen ZP, Watts MR, Kodama K, Carr DB, Utzschneider KM, Zraika S, Wang F, Kahn SE. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005;54:2235–2244. doi: 10.2337/diabetes.54.7.2235. [DOI] [PubMed] [Google Scholar]


