Abstract
The extracellular calcium (Ca2+o)-sensing receptor (CaR) recognizes and responds to (i.e., “senses”) Ca2+o as its principal physiological ligand. In the present studies, we document that the CaR is activated not only by extracellular calcium ions but also by amino acids, establishing its capacity to sense nutrients of two totally different classes. l-Amino acids, especially aromatic amino acids, including l-phenylalanine and l-tryptophan, stereoselectively mobilized Ca2+ ions in the presence of the CaR agonists, Ca2+o, gadolinium (Gd3+o), and spermine in fura-2-loaded human embryonic kidney (HEK-293) cells stably transfected with the human CaR. l-amino acid-dependent effects were observed above, but not below, a threshold level of Ca2+o of approximately 1.0 mM. l-Amino acids, particularly aromatic amino acids, also stereoselectively enhanced the sensitivity of the CaR to its agonists, Ca2+o and spermine. Branched-chain amino acids were almost inactive, and charged amino acids, including arginine and lysine, were much less effective than aromatic and other amino acids. l-amino acid mixtures emulating the amino acid composition of fasting human plasma reproduced the effects of high concentrations of individual l-amino acids on Ca2+ mobilization and enhanced the sensitivity of the CaR to Ca2+o. The data presented herein identify the CaR as a molecular target for aromatic and other l-amino acids. Thus, the CaR can integrate signals arising from distinct classes of nutrients: mineral ions and amino acids. The actions of l-amino acids on the CaR may provide explanations for several long recognized but poorly understood actions of dietary protein on calcium metabolism.
Biological sensing of l-amino acids plays key roles in coupling changes in whole body protein and amino acid metabolism to appropriate physiological responses. In the gut, the release of amino acids, especially aromatic amino acids such as phenylalanine and tryptophan, couples protein ingestion to appropriate responses such as gastric acid (1–3) and pancreatic enzyme (4, 5) release. However, the molecular identities of the receptors responsible for amino acid sensing in the gut, as well as in endocrine and other tissues, remain largely unknown. The extracellular calcium (Ca2+o)-sensing receptor (CaR; ref. 6) is homologous to metabotropic receptors for the amino acid glutamate but is activated by Ca2+o or other polyvalent cations. Its widespread distribution points to roles beyond those mediating calcium homeostasis, e.g., as a negative regulator of parathyroid hormone (PTH) release and as an activator of urinary Ca2+ excretion. For example, it is distributed widely in the gastrointestinal tract (7–9), including antral G cells where its activation stimulates gastrin release (10). Interactions between the CaR and amino acid metabolism are suggested by recent data showing marked elevation of PTH levels during protein restriction (11) and long-standing data showing that high protein intake promotes urinary calcium excretion (12, 13). Furthermore, an agonist binding site on the CaR was defined by the uncharged phenylalkylamine compound NPS R-467, which stereoselectively potentiates the actions of Ca2+o and other polycationic agonists on the receptor (14). This compound, which has structural similarity to phenylalanine and tyrosine, identifies a class of therapeutic activators of the CaR that are currently in clinical trials for treating hyperparathyroidism. On the basis of these considerations, we tested the effects of phenylalanine and other amino acids on the activity of the CaR. Herein, we show that aromatic and other l-amino acids stereoselectively activate the cloned human CaR in the presence of its polycationic agonists. These findings lead us to identify this receptor as a widely distributed l-amino acid sensor and to conclude that sensing of Ca2+ ions by the CaR must be considered in the context of the ambient level of amino acids. These data have implications for calcium homeostasis, gut physiology, and nutrient metabolism and may provide new insights into the neurotoxicity that arises from elevated levels of aromatic l-amino acids (e.g., in phenylketonuria).
Methods
Culture of HEK-293 Cells.
HEK-293 cells that had been stably transfected with the cloned human CaR (HEK-5001 cells) and untransfected HEK-293 cells were cultured in DMEM plus 10% (vol/vol) FBS under standard conditions (5% CO2; 37°C).
Amino Acid Solutions.
Stock amino acid-containing solutions were made up routinely in physiological saline at 100 or 200 mM with the exception of tryptophan (made up at 50 mM because of its poor solubility). The l-Cys stock was made up fresh daily as the free base l-cysteine. Tyrosine was difficult to retain in solution at neutral pH. The data shown in Table 1 were obtained from freshly prepared l-tyrosine solutions that were adjusted to pH 7.4 and used within 30 min of preparation at 37°C. The amino acid composition of the solution (termed 1× solution) that emulated the fasting levels of the amino acids in human plasma (all l-isomers in physiological saline solution; pH 7.4) was as follows (in μM): 50 Phe/50 Trp/80 His/60 Tyr/30 Cys/300 Ala/200 Thr/50 Asn/600 Gln/125 Ser/30 Glu/250 Gly/180 Pro/250 Val/30 Met/10 Asp/200 Lys/100 Arg/75 Ile/150 Leu (3, 15, 16). Concentrated stocks of this solution (20- or 40-fold higher than the final concentration of the respective amino acid) were made up daily in a physiological saline solution described in the next section and diluted in amino acid-free physiological saline as required.
Table 1.
Effect of l-amino acids on the potency of Ca2+ ions as agonists of the Ca2+ receptor
| Amino acid (10 mM) | EC50 for Ca2+ (n) | Δ EC50 for Ca2+ (n) |
|---|---|---|
| l-His | 2.4 ± 0.1 (4) | 1.9 ± 0.2 (4) |
| l-Phe | 2.5 ± 0.1 (7) | 1.8 ± 0.2 (7) |
| l-Tyr | 2.5 ± 0.2 (3) | 1.8 ± 0.2 (3) |
| l-Trp | 2.6 ± 0.3 (3) | 1.6 ± 0.2 (3) |
| l-Cys | 2.8 ± 0.1 (5) | 1.6 ± 0.1 (5) |
| l-Ala | 2.9 ± 0.3 (3) | 1.4 ± 0.04 (3) |
| l-Thr | 3.0 ± 0.1 (3) | 1.1 ± 0.3 (3) |
| l-Asn | 3.1 ± 0.2 (3) | 1.1 ± 0.2 (3) |
| l-Gln | 3.2 ± 0.2 (3) | 1.0 ± 0.2 (3) |
| l-Ser | 3.3 ± 0.2 (3) | 1.0 ± 0.2 (3) |
| l-Glu | 3.5 ± 0.2 (3) | 0.9 ± 0.1 (3) |
| Gly | 3.6 ± 0.5 (3) | 0.7 ± 0.2 (3) |
| l-Pro | 3.6 ± 0.3 (3) | 0.6 ± 0.2 (3) |
| l-Val | 3.6 ± 0.3 (3) | 0.6 ± 0.2 (3) |
| l-Met | 3.6 ± 0.3 (3) | 0.6 ± 0.1 (3) |
| l-Asp | 3.7 ± 0.2 (3) | 0.6 ± 0.3 (3) |
| l-Lys | 3.7 ± 0.1 (3) | 0.6 ± 0.2 (3) |
| l-Arg | 3.7 ± 0.1 (3) | 0.5 ± 0.3 (3) |
| l-Ile | 3.9 ± 0.5 (3) | 0.3 ± 0.1 (3) |
| l-Leu | 4.2 ± 0.3 (3) | 0 ± 0.2 (3) |
| Control | 4.2 ± 0.1 (10) | 0 ± 0 |
Data are shown ± SEM. All experiments were performed at pH 7.4. Δ EC50 is defined as control minus experimental. Unpaired t tests performed on the data set (individual amino acids vs. control) yielded the following results: l-His, l-Phe, l-Trp, l-Tyr, l-Cys, and l-Thr, P = 0.0001; l-Ala, P = 0.0002; l-Asn, l-Gln, l-Ser, and l-Glu, P ≤ 0.01; Gly, l-Pro, l-Val, l-Met, l-Asp, and l-Arg, P < 0.05; l-Lys, P = 0.05; l-Ile, P = 0.31; and l-Leu, P = 0.95.
Microfluorimetry for Determining Cytoplasmic Ca2+ Concentration.
For microfluorometry experiments, HEK-5001 cells were cultured on glass coverslips in six-well plates and loaded with fura-2AM (5 μM; 2 h; 37°C) in a solution of the following composition: 125 mM NaCl/4.0 mM KCl/1.25 mM CaCl2/1.0 mM MgSO4/20 mM Hepes (NaOH)/1 mM Na2HPO4/0.1% d-glucose and 0.1% BSA (pH 7.4) as described previously (17). Coverslips were washed with fura-2-free solution and preincubated for 30 min before experiments. The control physiological saline used in all microfluorometry experiments had the following composition: 125 mM NaCl/4.0 mM KCl/0.5 mM CaCl2/0.5 mM MgCl2/20 mM Hepes (NaOH)/0.1% d-glucose, pH 7.4. Coverslips were inserted diagonally into modified 1-cm fluorometry cuvettes above a Teflon-coated stirring bar, and the cuvettes were inserted into the light-path of a Delta Scan-1 microfluorometer (Photon Technology International, Princeton) where they were stirred continuously and maintained at 37°C. Excitation at alternating wavelengths (340 nm and 380 nm), detection of emitted light (peak 510 nm), and its digitized recording with deltascan software on a PC running Windows 95 were performed as described (17). Data for cytoplasmic Ca2+ concentration (Ca2+i) were either expressed as uncorrected fluorescence ratios (340/380) or converted to Ca2+i (in nM) by using a calibration procedure (18). Baseline Ca2+i was typically about 40 nM and rose approximately 10-fold on maximal stimulation of the CaR. Data are generally presented as fluorescence ratios (Figs. 1–5). Calibrated data were used to calculate the fractional cytoplasmic Ca2+ responses at various Ca2+o and l-Phe concentrations (Fig. 4c) as well as the EC50 values for Ca2+o in the presence of various l-amino acids (Table 1) and the decrease in the EC50 values for Ca2+o caused by adding amino acids (Table 1 and Fig. 4d). All data are expressed as means ± SEM (number of experiments). Curve fitting to the Hill equation was performed by using deltagraph software for Macintosh. All experiments described herein were performed a minimum of three times.
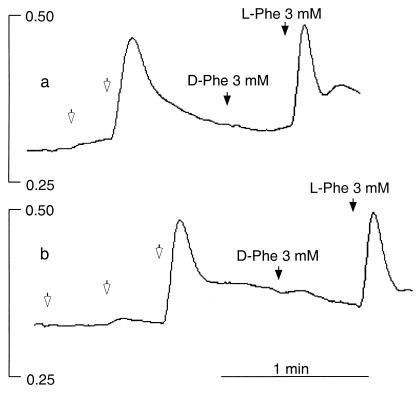
Figure 1.
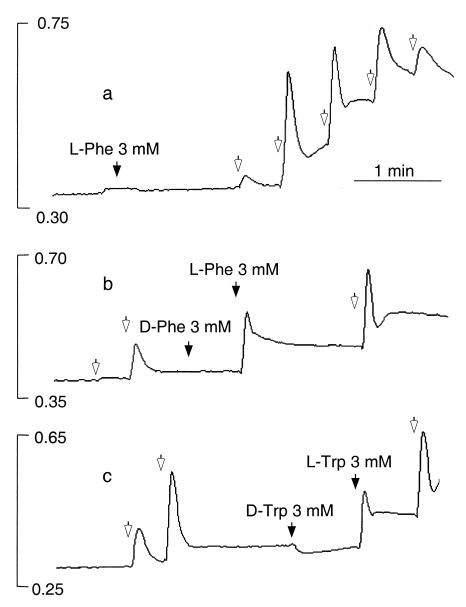
Effects of extracellular Ca2+, l-Phe, and l-Trp on Ca2+ mobilization in HEK-293 cells stably expressing the human CaR. HEK-5001 cells growing on glass coverslips were loaded with fura-2 and exposed to test agents in a stirred fluorescence cuvette at 37°C in the light path of a fluorometer. In all three panels, baseline Ca2+o was 0.5 mM, and 1-mM increments of Ca2+o were applied at the open arrows. The vertical bars indicate the fluorescence ratios (340/380). (a) Lack of effect of 3 mM l-Phe (closed arrow) at basal (subthreshold) Ca2+o (0.5 mM) and effect of repetitive 1-mM increments of Ca2+o (open arrows). (b) Stereoselective mobilization of Ca2+ by 3 mM l-Phe but not 3 mM d-Phe (closed arrows) at 2.5 mM Ca2+o. (c) Stereoselective mobilization of Ca2+ by 3 mM l-Trp but not 3 mM d-Trp (closed arrows) at 2.5 mM Ca2+o.
Figure 5.
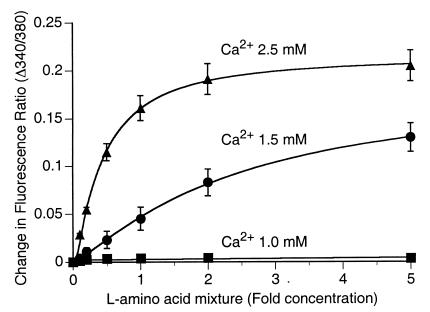
Concentration-dependent effects of l-amino acid mixtures on Ca2+ mobilization and sensitivity of the CaR to Ca2+o. HEK-5001 cells were exposed to physiological saline in the absence or presence of various fold (0- to 5-fold) concentrations of a mixture that emulated the fasting plasma levels of the 20 common l-amino acids. Concentration-dependent Ca2+ mobilization by the l-amino acid mixture was observed at 1.5 mM Ca2+o (●) and 2.5 mM Ca2+o (▴) but not 1.0 mM Ca2+o (■). The data (means ± SEM; n = 4) were derived from cumulative cytoplasmic Ca2+ responses (in the form of increased 340/380 fluorescence ratios).
Figure 4.
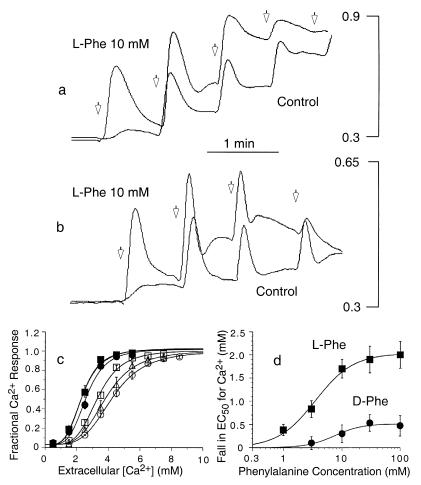
Modulatory effect of l-Phe on Ca2+ mobilization induced by Ca2+o and spermine. Fura-2-loaded HEK-293 cells that stably expressed the CaR (HEK-5001 cells) were exposed to physiological saline in the absence or presence of l-Phe or d-Phe before being loaded into the fluorometer. Baseline Ca2+o was 0.5 mM. The vertical bars in a and b indicate the fluorescence ratios (340/380). (a) Effects of 1-mM increments of Ca2+o (open arrows) on Ca2+ mobilization in the presence or absence of 10 mM l-Phe. (b) Effects of 0.25-mM increments of spermine (open arrows) on Ca2+ mobilization in the presence or absence of 10 mM l-Phe. (c) Effects of various concentrations of l-Phe on the fractional response of the CaR to Ca2+o. l-Phe concentrations were as follows: ○, zero; ▵, 1.0 mM; □, 3.0 mM; ●, 10 mM; ▴, 30 mM; ■, 100 mM. (d) Concentration dependence of the stereoselective decrease in the EC50 for Ca2+o induced by l-Phe. The following parameters were obtained by curve fitting for l-Phe: EC50 = 3.5 mM; Hill coefficient = 1.4. The following parameters were obtained by curve fitting for d-Phe: EC50 = 7.4 mM; Hill coefficient = 1.9.
Materials
HEK-5001 cells were kindly provided by NPS Pharmaceuticals (Salt Lake City, UT). All reagents were analytical grade or equivalent. l- and d-amino acids were obtained from Sigma. Fura-2 AM was obtained from Molecular Probes. Cell culture media were obtained from Life Technologies (Grand Island, NY).
Results
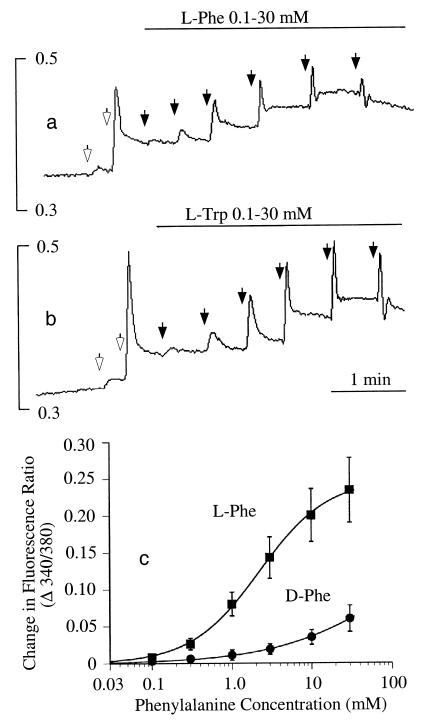
l-Amino acids had marked effects on Ca2+i in HEK-293 cells that stably expressed the cloned human CaR (HEK-5001 cells); these effects depended on the prevailing level of Ca2+o. At subthreshold Ca2+o (≤1.0 mM), l-amino acids (up to 3 mM) had no effect (Fig. 1a). However, at levels of Ca2+o above a threshold value between 1.0 and 1.5 mM (n = 4), l-Phe, l-Trp, and several other amino acids mobilized Ca2+ ions as indicated by transient elevations in Ca2+i. The effect was selective for l- rather than d-stereoisomers (Fig. 1 b and c), but weak agonist effects were observed at high concentrations (≥3 mM) of several d-amino acids, including d-Phe (Fig. 2c), d-Trp, d-His, and d-Ala (not shown). l-Amino acids (3 mM) that were effective in the presence of 2.5 mM Ca2+o included, in the order of their effectiveness, l-Phe = l-Trp = l-His ≥ l-Ala > l-Ser = l-Pro = l-Glu ≥ l-Asp but not l-Lys, l-Arg, l-Leu, and l-Ile. l-Phe and l-Trp exerted effects at concentrations as low as 0.1 mM (Fig. 2 a–c), and at 2.5 mM Ca2+o, the concentrations of l-Phe and l-Trp that induced half-maximal activation were approximately 2.5 mM in each case (Fig. 2 a–c). l-Phe also mobilized Ca2+ ions in the presence of submaximal concentrations of other CaR agonists, including spermine (Fig. 3a) and Gd3+o (Fig. 3b) under conditions where Ca2+o was held constant at 0.5 mM.
Figure 2.
Concentration-dependent mobilization of Ca2+ ions by l-Phe and l-Trp. In a and b, baseline Ca2+o was 0.5 mM, and 1-mM increments of Ca2+o were applied at the open arrows. The vertical bars in a and b indicate the fluorescence ratios (340/380). (a) Effect of repetitive increments of l-Phe (closed arrows, yielding the following final concentrations: 0.1, 0.3, 1.0, 3.0, 10, and 30 mM) in the presence of 2.5 mM Ca2+o. (b) Effect of repetitive increments of l-Trp (closed arrows, yielding the following final concentrations: 0.1, 0.3, 1.0, 3.0, 10, and 30 mM) in the presence of 2.5 mM Ca2+o. (c) Concentration-dependent activation of Ca2+ mobilization by l-Phe and d-Phe at 2.5 mM Ca2+o. The data were derived from cumulative Ca2+i responses (in the form of increased 340/380 fluorescence ratios). The curves were fitted by using the Hill equation. Parameters obtained for l-Phe included EC50 (2.2 mM) and the Hill coefficient (1.0).
Figure 3.
Effect of l-Phe on Ca2+ mobilization in the presence of submaximal concentrations of the polycationic CaR agonists spermine and Gd3+o. Ca2+o remained at subthreshold levels (0.5 mM) throughout these experiments. Increments of spermine (0.25 mM) and Gd3+o (5 μM) were applied at the open arrows. The vertical bars indicate the fluorescence ratios (340/380). (a) Stereoselective mobilization of Ca2+ by l-Phe but not d-Phe (closed arrows) after exposure of HEK-5001 cells to repetitive 0.25-mM increments of spermine. (b) Stereoselective mobilization of Ca2+ by l-Phe but not d-Phe (closed arrows) after repetitive 5-μM increments of Gd3+o.
HEK-5001 cells, like HEK-293 cells, also express phospholipase C coupled P2Y1 and P2Y2 purinoceptors (19), muscarinic receptors (20), and thrombin receptors (17). After exposure of HEK-5001 cells to the thrombin receptor agonist SFLLRN-amide (3 μM) or carbachol (1–10 μM), which yielded comparable cytoplasmic Ca2+ responses to the response induced by 2.5 mM Ca2+o, no effects were observed in response to several amino acids (all 3 mM) that were active in the presence of CaR agonists, including l-Phe, l-Trp, l-Ala, and Gly; the inactive amino acid l-Leu was also ineffective under these conditions (not shown). However, after exposure of HEK-5001 cells to submaximal concentrations of ATP (0.3–3 μM), transient elevations in [Ca2+]i were observed in response to several amino acids. The order of effectiveness at 3 mM was l-Ala = Gly > l-Leu ≥ l-Phe > l-Trp = 0. Thus, the efficacies of these amino acids for modulating P2Y-mediated and CaR-mediated cytoplasmic Ca2+ responses were clearly different. We also examined the behavior of untransfected HEK-293 cells. As expected for these cells, which do not express the CaR, there was no response to elevated Ca2+ in the absence or presence of l-amino acids. In the presence of submaximal concentrations of ATP, however, we observed similar responses to l-amino acids and a similar order of effectiveness to that observed after exposure to ATP in HEK-5001 cells.
We also examined whether l-amino acids might modulate the CaR by increasing its sensitivity to its divalent and polycationic activators. Active amino acids left-shifted the concentration response curves for agonist-induced Ca2+ mobilization with the agonists Ca2+o (Fig. 4a) and spermine (Fig. 4b). As identified above in the case of Ca2+ mobilization, the aromatic amino acids, l-Phe, l-Trp, l-Tyr, and l-His (neutral at pH 7.4), were the most effective compounds when assayed for their abilities to left-shift the concentration response curve for extracellular Ca2+ (Table 1). l-Phe shifted the concentration response curve in a concentration-dependent manner (Fig. 4c) and maximally lowered the EC50 for Ca2+o as a CaR agonist from 4.2 ± 0.2 mM to 2.2 ± 0.1 mM (n = 4). l-Phe was markedly more effective than d-Phe in lowering the EC50 for Ca2+o (Fig. 4d); the half-maximal effect of l-Phe was observed at a concentration of 3.5 mM. The order of effectiveness (at 10 mM) with respect to enhanced receptor sensitivity to Ca2+o (pH 7.4) was l-His = l-Phe = l-Tyr = l-Trp ≥ l-Cys = l-Ala = l-Thr ≥ l-Asn = l-Gln = l-Ser ≥ l-Glu = Gly = l-Pro = l-Val = l-Met ≥ l-Asp = l-Lys = l-Arg (Table 1). l-Ile and l-Leu were ineffective. As a class, therefore, branched-chain amino acids were strikingly less effective than aromatic amino acids. d-His, d-Trp, and d-Ala, like d-Phe, had weak modulatory activity compared with their respective l-analogs, but the effects of d-Ser and l-Ser were approximately equivalent, consistent, in part, with data reported for gastrin release in dogs (21). We also examined the effect of the glutamate analog γ-aminobutyric acid (GABA). At 10 mM, GABA had no effect on CaR function.
We next examined the structural requirements for CaR activation by using the small, active neutral amino acid l-Ala. The CaR had marked stereoselectivity for l-Ala vs. d-Ala similar to that observed for l-Phe and l-Trp (not shown). In addition, the following structural analogs of l-Ala were either much less effective or inactive when tested as activators of the CaR at a concentration of 10 mM: β-alanine, propionate, l-lactate, l-pyruvate, and ethylamine. These data indicate that receptor activation by l-amino acids requires both the α-amino and carboxyl groups as well as an appropriate side chain.
To investigate the physiological significance of our findings further, we examined whether mixtures of the 20 common l-amino acids at levels approximating those present in fasting human plasma could reproduce the effects of high concentrations of single amino acids. HEK-293 cells stably expressing the human CaR (HEK-5001 cells) were incubated in physiological saline solutions that contained 0.1–5× the published fasting levels of l-amino acids in human plasma (3, 15, 16). At Ca2+o ≤ 1.0 mM, l-amino acid mixtures had no effect on Ca2+i in fura-2-loaded HEK-5001 cells. However, above a threshold between 1.0 and 1.5 mM (n = 4), l-amino acid mixtures induced concentration-dependent transient elevations in Ca2+i similar to those observed for individual l-amino acids (Fig. 5). At 1.5 mM Ca2+o, which is close to the level of ionized Ca2+ normally present in human plasma, the CaR was sensitive to variations in fold concentration of the l-amino acid mixture that correspond to those normally observed under conditions of protein restriction or high protein intake (approximately 0.5–2×), and this sensitivity was enhanced further at 2.5 mM Ca2+o. In addition, l-amino acid mixtures increased the sensitivity of the CaR to Ca2+o. The EC50 for Ca2+o fell maximally from 4.2 ± 0.2 mM (n = 7) to 2.4 ± 0.1 mM (n = 4; P = 0.0001), and the half-maximal fall in the EC50 for Ca2+o was observed at ≈0.5× the normal fasting amino acid content (not shown). These findings indicate that mixtures of l-amino acids are indeed capable of concerted action on the CaR. Together with the data in Table 1, which show significant responses to 18 of the 20 common l-amino acids, these data indicate that the CaR senses the total level of free l-amino acids and that the amino acid composition of human plasma has a pronounced effect on the CaR's sensitivity to its polycationic agonists.
Discussion
The finding that the CaR can also behave as a stereoselective receptor for l-amino acids draws it closer functionally to its most closely related homologs, the metabotropic glutamate receptors and the GABAB receptors for the glutamate metabolite GABA. Metabotropic glutamate receptors are activated by the negatively charged amino acid glutamate in the submillimolar range, and as in this study, several isoforms have been shown recently to have positive cooperativity between the amino acid activator and Ca2+ ions (22). The CaR is, however, the only member of this receptor family identified thus far to select for aromatic l-amino acids and to respond more generally to a large number of l-amino acids. It is of interest that the large amino-terminal extracellular domains of the homologous CaR, metabotropic glutamate receptors, GABAB, and putative pheromone receptors are thought to be structurally related to the bacterial periplasmic binding proteins (23), some of which bind amino acids. The data reported in the present study, therefore, would seem to provide additional evidence for this evolutionary link. However, it should be noted that the location of the l-amino acid binding site has not yet been determined and that data indicate that the binding site for phenylalkylamine activators of the CaR such as NPS R-467 resides in the transmembrane rather than the amino-terminal head region of CaR (24, 25). Whether l-amino acids share a common binding site with NPS R-467 or whether there is overlap between the binding sites for NPS R-467 and the amino acid activators of the CaR is not yet clear.
The interactions between l-amino acids and the CaR identified herein were not limited solely to this receptor. Some l-amino acids, including the aliphatic amino acids l-Ala and Gly and the branched-chain amino acid l-Leu but not the aromatic amino acids l-Phe and l-Trp, were effective modulators of P2Y purinoceptors. Although this pharmacological profile was clearly distinct from that shown by the CaR, the findings indicate that amino acid sensing is a property of certain G protein-coupled receptors (including perhaps receptors coupled to other signaling mechanisms, e.g., to adenylyl cyclase) and can provide a mechanism by which cells respond to changes in metabolic state.
Two basic modes of CaR operation were differentiated with respect to its activators in these experiments. First, if Ca2+o is constant and above threshold (>1.0 mM), the CaR can operate as an l-amino acid receptor. Second, when the free l-amino acid concentration is constant or in the total absence of amino acids, the CaR senses and responds to Ca2+ ions as previously recognized. In this latter case, occupancy of the l-amino acid binding site increases the sensitivity of the CaR to Ca2+o. It seems probable, however, that under many physiological conditions, the CaR integrates more complex signals arising from changing levels of both Ca2+ and amino acids. For example, after dietary ingestion of meat or milk, a rise in Ca2+ levels in both gut and subsequently plasma is accompanied by a corresponding load of amino acids released from the ingested protein. Concomitant changes in amino acid and Ca2+ levels like this change would seem to yield a third mode of operation in which Ca2+ and amino acids act as coagonists of the CaR.
The scope of these effects would seem to include the gastrointestinal tract as well as tissues bathed by portal and systemic blood. In the gastrointestinal tract, amino acid stimulation of CaRs may provide a “missing link” between the release of amino acids from ingested protein and the stimulation of gastric and pancreatic secretory responses. Consistent with this hypothesis, gastrin-secreting antral G cells (10) and gastric parietal cells (9), which express the CaR on their apical membranes, as well as cholecystokinin-secreting small intestinal I cells, which have not yet been examined for expression of this receptor, are exposed to total free amino acid levels that rise from fasting levels of around 2 mM to as high as 20–30 mM after a protein-containing meal (26, 27). Our data indicate that the CaR is capable of sensing and responding to changes in amino acid concentration within this range (Figs. 2, 4, and 5). The concept of amino acid activation of the CaR might also allow for an indirect link between protein ingestion and pancreatic enzyme release via intestinal cholecystokinin-releasing peptides (for review, see ref. 28). Strong immunostaining for the CaR in the enteric nervous system (8, 9), for example, may provide a link to cholecystokinin-releasing peptides that are synthesized and released by enteric neurons. In addition, after their absorption, dietary amino acids may promote gastric acid secretion and pancreatic enzyme secretion via CaRs on the basolateral membranes of gastric parietal cells (9) and pancreatic acinar cells (29). Such an action would be consistent, for example, with the known effects of i.v. amino acids on gastric acid secretion (2, 30).
The finding that the Ca2+ sensitivity of the CaR was enhanced by physiological amino acid mixtures in the total concentration range of 2–5 mM (Fig. 5), which corresponds to the range observed in systemic blood under extremes of dietary manipulation, has implications for Ca2+o-sensing by internal organs and tissues that express high levels of the CaR such as the parathyroid and kidney. The effect of l-amino acids on the Ca2+ sensitivity of the CaR was significant at levels found in fasting plasma (1× solution; total concentration of ≈3 mM) and enhanced further by elevations in total concentration similar to those that occur in plasma over several hours after a protein-containing meal (3, 16). Variations in dietary protein intake are known to have marked effects on calcium homeostasis, but the mechanisms involved have been unclear. The findings of this study provide potential explanations for several of these observations via variations in plasma levels of free amino acids. For example, amino acids may contribute to the phenomenon of elevated urinary calcium excretion in human subjects on high protein diets (12, 13). This action could arise either directly from the activation of CaRs on cells of the renal cortical thick ascending limb, which promote calcium excretion (31), or indirectly via amino acid-induced inhibition of PTH secretion by the parathyroid, because a reduction in PTH would also enhance urinary calcium excretion. In a similar manner, the observation that circulating PTH and calcitriol levels are elevated in young women undergoing protein restriction in the absence of changes in plasma total or ionized Ca2+ concentration (11) may be explained by a decrease in the activity of parathyroid CaRs in response to reduced levels of l-amino acids. This mechanism may also contribute to secondary hyperparathyroidism in patients with chronic renal failure who are on protein-restricted diets.
These findings may also shed light on the mechanisms that underlie aromatic amino acid-induced neurotoxicity, e.g., hepatic encephalopathy—in which an elevation of the ratio of the plasma levels of aromatic to branched-chain amino acids contributes to the disturbance in mental state—and phenylketonuria. In phenylketonuria, phenylalanine-induced central nervous system (CNS) toxicity arises from l-Phe concentrations ≥ 0.2 mM (i.e., ≥40× normal) in the cerebrospinal fluid, which interfere with normal CNS myelination in the postnatal period leading to leukodystrophy and an associated moderate to severe mental retardation in neonates fed a normal, phenylalanine-containing diet. The finding that the CaR is activated at concentrations of l-Phe exceeding 0.1 mM in the present study, together with the demonstration that the CaR is expressed at high levels on CNS oligodendrocytes (32) that synthesize myelin, leads us to speculate that inappropriate overstimulation of CaRs on oligodendrocytes by high concentrations of l-Phe may be responsible for the CNS toxicity in phenylketonuria.
It should be stressed that the hypotheses above relating to possible roles of the CaR in transducing signals from dietary protein intake on the one hand and aromatic amino acid neurotoxicity on the other are, at present, a matter of speculation and will require more direct, physiologically relevant studies before any firm conclusions can be drawn. If these predictions are confirmed, however, and if amino acid-induced dysfunction of the CaR is found to occur in parathyroid, kidney, gut, CNS, and/or other tissues, it may be possible to develop CaR activators or inhibitors that interact with the receptor's amino acid binding site(s), thereby permitting new therapeutic approaches to these conditions.
Acknowledgments
The authors thank Prof. R. M. Case for a critical reading of the manuscript. This work was supported by National Institutes of Health Grant NIH DK41415. A.D.C. was supported on study leave by the University of Sydney, New South Wales, Australia, and by the award of a Servier Fellowship in Metabolic Medicine from The Royal Australasian College of Physicians.
Abbreviations
- Ca2+o
extracellular calcium
- Ca2+i
cytoplasmic calcium
- CaR
Ca2+o-sensing receptor
- PTH
parathyroid hormone
- GABA
γ-aminobutyric acid
- CNS
central nervous system
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 4419.
References
- 1.Strunz U T, Walsh J H, Grossman M I. Proc Soc Exp Biol Med. 1978;157:440–441. doi: 10.3181/00379727-157-40072. [DOI] [PubMed] [Google Scholar]
- 2.Isenberg J I, Maxwell V. N Eng J Med. 1978;298:27–29. doi: 10.1056/NEJM197801052980106. [DOI] [PubMed] [Google Scholar]
- 3.McArthur K E, Isenberg J I, Hogan D L, Dreier S J. J Clin Invest. 1983;71:1254–1262. doi: 10.1172/JCI110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C C, Grossman M I. Am J Physiol. 1951;164:527–545. doi: 10.1152/ajplegacy.1951.164.2.527. [DOI] [PubMed] [Google Scholar]
- 5.Meyer J H, Grossman M I. Am J Physiol. 1972;222:1058–1063. doi: 10.1152/ajplegacy.1972.222.4.1058. [DOI] [PubMed] [Google Scholar]
- 6.Brown E M, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger M A, Lytton J, Hebert S C. Nature (London) 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 7.Gama L, Baxendale-Cox L M, Breitwieser G E. Am J Physiol. 1997;273:C1168–C1175. doi: 10.1152/ajpcell.1997.273.4.C1168. [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert S C, Soybel D I, Brown E M. Am J Physiol. 1998;274:G122–G130. doi: 10.1152/ajpgi.1998.274.1.G122. [DOI] [PubMed] [Google Scholar]
- 9.Cheng I, Qureshi I, Chattopadhyay N, Qureshi A, Butters R R, Hall A E, Cima R R, Rogers K V, Hebert S C, Geibel J P, et al. Gastroenterology. 1999;116:118–126. doi: 10.1016/s0016-5085(99)70235-0. [DOI] [PubMed] [Google Scholar]
- 10.Ray J, Squires P, Curtis S, Meloche M, Buchan A. J Clin Invest. 1997;99:2328–2333. doi: 10.1172/JCI119413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerstetter J E, Caseria D M, Mitnick M E, Ellison A F, Gay L F, Liskov T A P, Carpenter T O, Insogna K L. Am J Clin Nutr. 1997;66:1188–1196. doi: 10.1093/ajcn/66.5.1188. [DOI] [PubMed] [Google Scholar]
- 12.Johnson N E, Alcantara E N, Linkswiler H. J Nutr. 1970;100:1425–1430. doi: 10.1093/jn/100.12.1425. [DOI] [PubMed] [Google Scholar]
- 13.Allen L H, Oddoye E A, Margen S. Am J Clin Nutr. 1979;32:741–749. doi: 10.1093/ajcn/32.4.741. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth E F, Steffey M E, Hammerland L G, Hung B C P, van Wagenen B C, Delmar E G, Balandrin M F. Proc Natl Acad Sci USA. 1998;95:4040–4045. doi: 10.1073/pnas.95.7.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentner C. In: Geigy Scientific Tables. Lentner C, Lentner C, Wink A, editors. Basel: Ciba-Geigy; 1984. pp. 89–106. [Google Scholar]
- 16.Brand H S, Jorning G G A, Chamuleau R A F M, Abraham-Inpijn L. Clin Chim Acta. 1997;264:37–47. doi: 10.1016/s0009-8981(97)00070-3. [DOI] [PubMed] [Google Scholar]
- 17.Quinn S J, Kifor O, Trivedi S, Diaz R, Vassilev P, Brown E M. J Biol Chem. 1998;273:19579–19586. doi: 10.1074/jbc.273.31.19579. [DOI] [PubMed] [Google Scholar]
- 18.Quinn S J, Williams G H, Tillotson D L. Am J Physiol. 1988;255:E488–E495. doi: 10.1152/ajpendo.1988.255.4.E488. [DOI] [PubMed] [Google Scholar]
- 19.Schachter J B, Sromek S M, Nicholas R A, Harden T K. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 20.Vogler O, Krummenerl P, Schmidt M, Jakobs K H, Koppen C J V. J Pharmacol Exp Ther. 1999;288:36–42. [PubMed] [Google Scholar]
- 21.Csendes A, Grossman M I. Experientia. 1972;28:1306–1307. doi: 10.1007/BF01965311. [DOI] [PubMed] [Google Scholar]
- 22.Kubo Y, Miyashita T, Murata Y. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- 23.O'Hara P J, Sheppard P O, Thogersen H, Venezia D, Haldeman B A, McGrane V, Houamed K M, Thomsen C, Gilbert T L, Mulvihill E R. Neuron. 1993;11:41–52. doi: 10.1016/0896-6273(93)90269-w. [DOI] [PubMed] [Google Scholar]
- 24.Hammerland L G, Garrett J E, Krapcho K J, Simin R, Capuano I V, Alasti N, Nemeth E F, Fuller F H. J Bone Miner Res. 1996;11:P272. [Google Scholar]
- 25.Nemeth E F. In: Principles of Bone Biology. Bilezikian J P, Raisz L G, Rodan G A, editors. San Diego: Academic; 1996. pp. 1019–1035. [Google Scholar]
- 26.Adibi S A, Mercer D W. J Clin Invest. 1973;52:1586–1594. doi: 10.1172/JCI107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman E J, Grossman M I. Am J Physiol. 1980;239:G493–G496. doi: 10.1152/ajpgi.1980.239.6.G493. [DOI] [PubMed] [Google Scholar]
- 28.Herzig K. Regul Pept. 1998;73:89–94. doi: 10.1016/s0167-0115(97)01062-8. [DOI] [PubMed] [Google Scholar]
- 29.Bruce J I, Yang X, Ferguson C J, Elliott A C, Steward M C, Case R M, Riccardi D. J Biol Chem. 1999;274:20561–20568. doi: 10.1074/jbc.274.29.20561. [DOI] [PubMed] [Google Scholar]
- 30.Konturek S J, Tasler J, Cieszkowski M, Jaworek J. Gastroenterology. 1978;75:817–824. [PubMed] [Google Scholar]
- 31.Brown E M, Hebert S C. Kidney Int. 1996;49:1042–1046. doi: 10.1038/ki.1996.152. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay N, Ye C P, Yamaguchi T, Kifor O, Vassilev P M, Nishimura R, Brown E M. Glia. 1998;24:449–458. [PubMed] [Google Scholar]