Abstract
The brain of a ferret showing abnormal neurologic signs was evaluated by histopathologic, histochemical, immunohistochemical, and ultrastructural examinations. Extensive neuronal vacuolation was observed. Since the brain was negative for protease-resistant protein prion (PrPres), it was concluded that this was not a case of transmissible spongiform encephalopathy.
Résumé
Vacuolation neuronale chez un furet adulte. Le cerveau d’un furet présentant des signes neurologiques anormaux a été évalué par des examens d’histopathologie, d’histochimie, d’immunohistochimie et d’ultrastructure. Une vacuolisation neuronale extensive a été observée. Comme le test aux protéines de prion résistantes aux protéases (PrPres) était négatif, il a été conclu qu’il ne s’agissait pas d’un cas d’encéphalopathie spongiforme transmissible.
(Traduit par Docteur André Blouin)
Since the outbreak of bovine spongiform encephalopathy (BSE) in the United Kingdom, there has been concern that the disease would occur in the United States. Although at the time of writing this report, only 3 cases of BSE (2 indigenous and 1 imported from Canada) have been diagnosed in this country, diagnostic laboratories need to be vigilant in recognizing lesions of transmissible spongiform encephalopathies (TSEs) or prion diseases.
Primary neurologic disorders are uncommon in pet ferrets (1). This communication describes presence of neuronal vacuolation in the brain of an adult ferret. A 5-year-old, spayed female pet ferret was presented to the referring veterinarian with a 3-day history of a rapidly progressive neurologic disorder, characterized by shivering, convulsions, and incoordination. The animal was treated with enrofloxacin and diazepam, as well as lactated Ringer’s solution, SC. Treatment did not result in clinical improvement and the animal was euthanized by the referring veterinarian. The carcass was submitted to the Iowa State University Veterinary Diagnostic Laboratory for further investigation.
The carcass was moderately fleshed. Autolysis was minimal. Significant gross lesions were not identified. Results of fluorescent antibody (FA) testing for Rabies virus on the hippocampus and caudal medulla were negative. Likewise, results of FA testing on brain and lung for Canine distemper virus were negative. Bacteria of significance were not isolated from lung, spleen, brain, or a meningeal swab. Representative samples of brain, lung, heart, spleen, liver, kidney, adrenal, stomach, intestine, and urinary bladder were immersion fixed in 10% buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (HE). Sections of brain were also stained with periodic acid-Schiff (PAS) and toluidine blue and immunohistochemically (IHC) for pathologic prion protein (PrPres). Two monoclonal antibodies, F89/160.1.5 and F99/97.6.1, were used in the IHC test. With each batch of tissues for IHC staining, sections of scrapie positive and a scrapie negative brainstem were included as controls. Selected areas of the formalin-fixed brain with lesions, seen in adjacent sections from the paraffin block, were postfixed with 1% osmium tetroxide to prevent removal of lipid material from the tissues during histologic processing, embedded in paraffin, sectioned at 5-μm, and stained with HE.
Selected areas of formalin-fixed brain with lesions, in adjacent sections from the paraffin block, were also placed first in 2.5% glutaraldehyde in cacodylate buffer, then in 1.0% osmium tetroxide in cacodylate buffer, before being embedded in epon-araldite mixture. Thick (1 μm) sections were stained with toluidine blue; then selected areas were thin sectioned, stained with saturated uranyl acetate and lead citrate, and examined with an electron microscope.
Rabies virus antigen and PrPres were not detected by FA and IHC, respectively. Histochemical stains and osmicated brain tissue stained by HE did not reveal the presence of stainable material within the vacuoles.
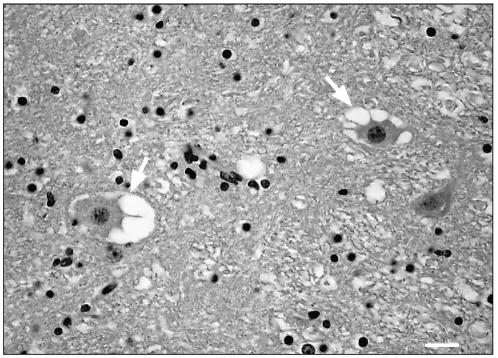
Under light microscopic examination, prominent single or multiple neuronal vacuolations were observed in the rostral medulla and cerebellar roof nuclei (Figure 1). The vacuoles were up to 40 μm in diameter and appeared to be empty. Similar but isolated vacuoles were also present in the neurons of the hippocampus and in Purkinje cells. Significant microscopic lesions were not observed in the other tissues.
Figure 1.
Brainstem. Several neurons with multiple clear vacuoles in neuronal perykaria (arrows). Hematoxylin and eosin, Bar = 25μm.
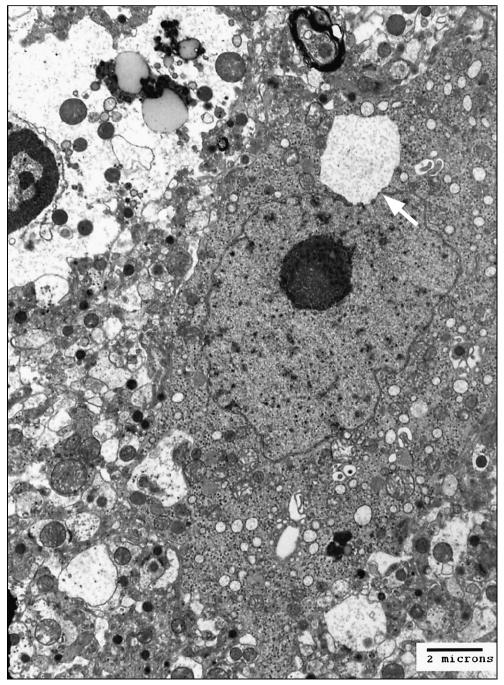
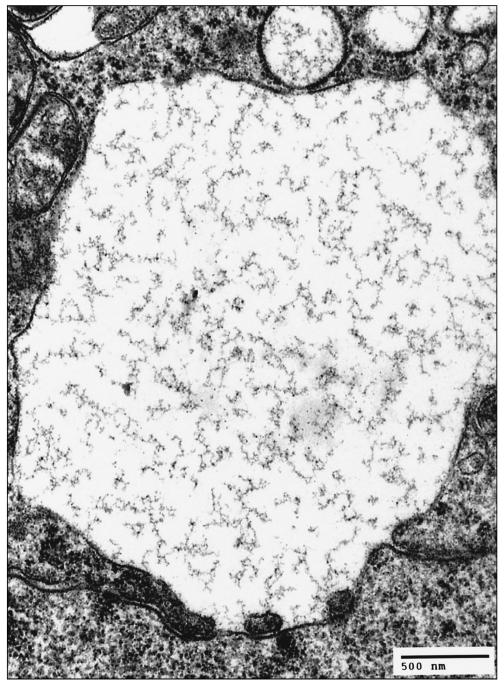
Ultrastructural examination of the formalin-fixed brainstem revealed fragmentation and vesiculation of the rough endoplasmic reticulum that resulted in many variably sized vacuoles distributed throughout the cytoplasm (Figure 2a), sometimes extending into neuronal processes. At higher magnification, the vacuoles showed loosely packed polymerized protein filaments (Figure 2b) and the membrane of the vacuole appeared to originate from the outer leaflet of the nuclear envelope.
Figure 2a.
Electron micrograph, cerebellar roof nucleus. The neuron shows a large cytoplasmic membrane bound vacuole (arrow) near the nucleus. Uranyl acetate and lead citrate.
Figure 2b.
Electron micrograph, cerebellar roof nucleus. Higher magnification of Figure 2a. The cytoplasmic vacuole shows loosely packed polymerized protein filaments. The membrane of the vacuole originates from the outer leaflet of the nuclear envelope. Uranyl acetate and lead citrate.
Although naturally occurring TSEs have not been documented in ferrets, transmissible mink encephalopathy (TME), a TSE, can be experimentally transmitted to this species when inoculated during fetal life (2). Also, a laboratory passaged strain of chronic wasting disease (CWD) of mule deer can be transmitted within a relatively short period (5 mo) when inoculated intracerebrally into ferrets (3). Therefore, it was essential to rule out the possibility of a TSE being the cause of the vacuolations in the brain of this ferret.
The histologic characteristics of TSEs are spongiform changes in neuropil and the presence of cytoplasmic vacuolation in neurons (4). However, not all cases of TSEs, especially those that are experimentally produced by cross-species transmissions, have resulted in manifestations of obvious spongiform encephalopathy (5). Additionally, the presence of vacuoles in neuronal perikaria has been demonstrated in non-TSE conditions of animals, such as rabies in skunks and fox (6) and in a heifer (7), in weak and ataxic Rottweiler pups (8), in young neurologically abnormal Angora goats (9), and in lysosomal and acquired storage diseases of various animals (10). An idiopathic case of widespread neuronal vacuolation has been described in a neonatal calf (11) and vacuolation due to cytoplasmic accumulation of lipid has recently been reported in raccoons from north western United States (12). To our knowledge, vacuolation of neurons has not been seen in the brain of ferrets with rabies.
In the presently described case, spongiform change in the neuropil, as well as vacuolation of the neuronal perikarya, was seen in various anatomic locations in the brain (brainstem, hippocampus, and cerebellum). Such findings would be suggestive not only of a TSE, but also of other pathological conditions. Identification of diseases and lesions with morphologic similarities to TSEs is important in the differential diagnosis of the disease. Apart from the 3 cases of BSE in cattle in the United States, there are many more cases of other TSEs in its animal populations, such as scrapie in sheep and goats, CWD in cervids, and TME in mink. Therefore, any suspicion of a TSE, however remote, has to be ruled out. In this ferret, the ultrastructural studies revealed membrane-bound cytoplasmic vacuoles, consistent with a primary neuronal degeneration. Since the confirmatory diagnostic test for PrPres was negative, it was concluded that this was not a case of TSE. However, although no natural cases of TSEs have been reported in ferrets to date, the possibility of diagnosing a new TSE in this species in the future cannot be ruled out.
Acknowledgments
We thank Dr. Cheville for assistance with the interpretation of electron micrographs and Dr. Katherine I. O’Rourke for providing the antibody for IHC. Judi Stasko for electron microscopy, and James Fossi for photomicrographs, Martha Church, Sharla Van Roekel, and Ginny Montgomery at NADC provided expert technical assistance.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. CVJ
References
- 1.Antinoff N. Musculoskeletal and neurologic diseases. In: Quesenberry KE, Carpenter JW, eds. Ferrets, Rabbits, and Rodents, 2nd ed. Missouri: Elsevier, 2004:115–120.
- 2.Eckroade RJ, Zu Rhein GM, Hanson RP. Transmissible mink encephalopathy in carnivores: Clinical, light and electron microscopic studies in raccoons, skunks and ferrets. J Wildl Dis. 1973;9:229–240. doi: 10.7589/0090-3558-9.3.229. [DOI] [PubMed] [Google Scholar]
- 3.Bartz JC, Marsh RF, McKenzie DI, Aiken JM. The host range of chronic wasting disease is altered on passage in ferrets. Virology. 1998;251:297–301. doi: 10.1006/viro.1998.9427. [DOI] [PubMed] [Google Scholar]
- 4.Hadlow WJ. Neuropathology and the scrapie-kuru connection. Brain Pathol. 1995;5:27–31. doi: 10.1111/j.1750-3639.1995.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamir AN, Kunkle RA, Cutlip RC. Experimental transmission of chronic wasting disease agent to cattle by intracerebral route. J Vet Diagn Invest. 2005;17:276–281. doi: 10.1177/104063870501700313. [DOI] [PubMed] [Google Scholar]
- 6.Charlton KM, Casey GA, Webster WA, Bundza A. Experimental rabies in skunks and foxes: Pathogenesis of the spongiform lesion. Lab Invest. 1987;57:634–645. [PubMed] [Google Scholar]
- 7.Foley GL, Zachary JF. Rabies-induced spongiform change and encephalitis in a heifer. Vet Pathol. 1995;32:309–311. doi: 10.1177/030098589503200313. [DOI] [PubMed] [Google Scholar]
- 8.Kortz GD, Meier WA, Higgins RJ, et al. Neuronal vacuolation and spinocerebellar degeneration in young Rottweiler dogs. Vet Pathol. 1997;34:296–302. doi: 10.1177/030098589703400405. [DOI] [PubMed] [Google Scholar]
- 9.Lancaster MJ, Gill IJ, Hooper PT. Progressive paresis in Angora goats. Aust Vet J. 1987;64:123–124. doi: 10.1111/j.1751-0813.1987.tb09652.x. [DOI] [PubMed] [Google Scholar]
- 10.Jubb KVF, Huxtable CR. The nervous system. In: Jubb KVF, Kennedy PC, Palmer N, eds. Pathology of Domestic Animals, San Diego: Acad Pr, 1993:267–439.
- 11.Hamir AN, Habecker P, Jenny A, et al. Idiopathic disseminated intra-cytoplasmic neuronal vacuolation in a neonatal Holstein calf born in USA. J Vet Diagn Invest. 2001;13:349–351. doi: 10.1177/104063870101300413. [DOI] [PubMed] [Google Scholar]
- 12.Hamir AN, Fischer KA. Neuronal vacuolations in raccoons from Oregon. J Vet Diagn Invest. 1999;11:303–307. doi: 10.1177/104063879901100401. [DOI] [PubMed] [Google Scholar]