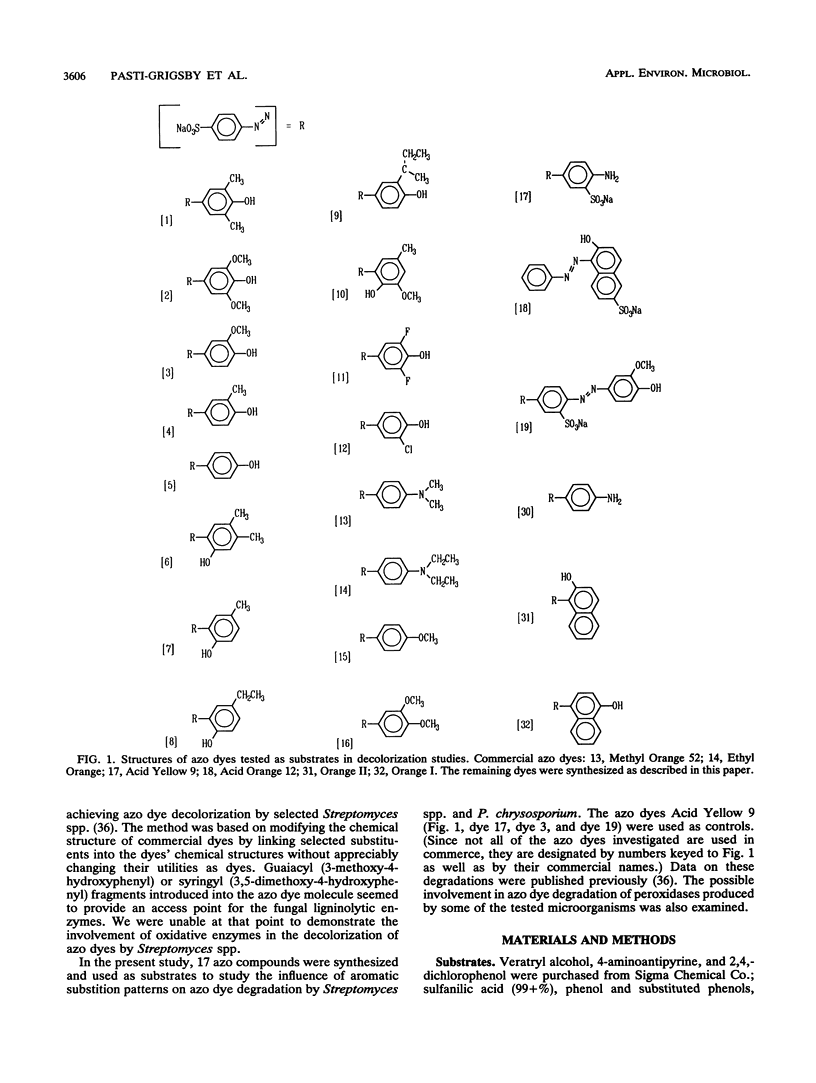
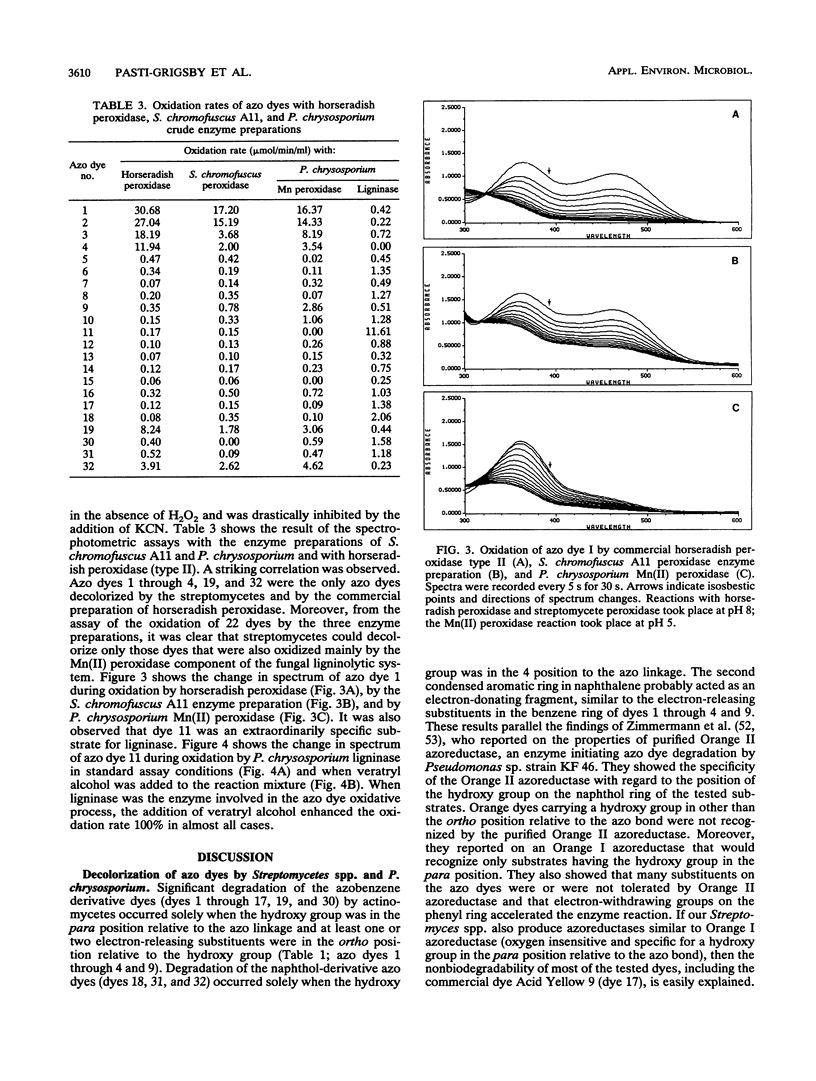
Abstract
Twenty-two azo dyes were used to study the influence of substituents on azo dye biodegradability and to explore the possibility of enhancing the biodegradabilities of azo dyes without affecting their properties as dyes by changing their chemical structures. Streptomyces spp. and Phanerochaete chrysosporium were used in the study. None of the actinomycetes (Streptomyces rochei A10, Streptomyces chromofuscus A11, Streptomyces diastaticus A12, S. diastaticus A13, and S. rochei A14) degraded the commercially available Acid Yellow 9. Decolorization of monosulfonated mono azo dye derivatives of azobenzene by the Streptomyces spp. was observed with five azo dyes having the common structural pattern of a hydroxy group in the para position relative to the azo linkage and at least one methoxy and/or one alkyl group in an ortho position relative to the hydroxy group. The fungus P. chrysosporium attacked Acid Yellow 9 to some extent and extensively decolorized several azo dyes. A different pattern was seen for three mono azo dye derivatives of naphthol. Streptomyces spp. decolorized Orange I but not Acid Orange 12 or Orange II. P. chrysosporium, though able to transform these three azo dyes, decolorized Acid Orange 12 and Orange II more effectively than Orange I. A correlation was observed between the rate of decolorization of dyes by Streptomyces spp. and the rate of oxidative decolorization of dyes by a commercial preparation of horseradish peroxidase type II, extracellular peroxidase preparations of S. chromofuscus A11, or Mn(II) peroxidase from P. chrysosporium. Ligninase of P. chrysosporium showed a dye specificity different from that of the other oxidative enzymes.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball A. S., Godden B., Helvenstein P., Penninckx M. J., McCarthy A. J. Lignocarbohydrate solubilization from straw by actinomycetes. Appl Environ Microbiol. 1990 Oct;56(10):3017–3022. doi: 10.1128/aem.56.10.3017-3022.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. P. Reduction of polymeric azo and nitro dyes by intestinal bacteria. Appl Environ Microbiol. 1981 May;41(5):1283–1286. doi: 10.1128/aem.41.5.1283-1286.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Brock B. J. Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 May;54(5):1143–1150. doi: 10.1128/aem.54.5.1143-1150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Franklin W., Pack L. D. Metabolism of benzidine and benzidine-congener based dyes by human, monkey and rat intestinal bacteria. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1224–1229. doi: 10.1016/s0006-291x(82)80128-9. [DOI] [PubMed] [Google Scholar]
- Chung K. T., Stevens S. E., Jr, Cerniglia C. E. The reduction of azo dyes by the intestinal microflora. Crit Rev Microbiol. 1992;18(3):175–190. doi: 10.3109/10408419209114557. [DOI] [PubMed] [Google Scholar]
- Crawford D. L., Crawford R. L. Microbial degradation of lignocellulose: the lignin component. Appl Environ Microbiol. 1976 May;31(5):714–717. doi: 10.1128/aem.31.5.714-717.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps C., Bumpus J. A., Aust S. D. Biodegradation of azo and heterocyclic dyes by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Apr;56(4):1114–1118. doi: 10.1128/aem.56.4.1114-1118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Decolorization of Several Polymeric Dyes by the Lignin-Degrading Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Jun;45(6):1741–1747. doi: 10.1128/aem.45.6.1741-1747.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel K. E., Kalyanaraman B., Kirk T. K. Oxidation of polycyclic aromatic hydrocarbons and dibenzo[p]-dioxins by Phanerochaete chrysosporium ligninase. J Biol Chem. 1986 Dec 25;261(36):16948–16952. [PubMed] [Google Scholar]
- Haug W., Schmidt A., Nörtemann B., Hempel D. C., Stolz A., Knackmuss H. J. Mineralization of the sulfonated azo dye Mordant Yellow 3 by a 6-aminonaphthalene-2-sulfonate-degrading bacterial consortium. Appl Environ Microbiol. 1991 Nov;57(11):3144–3149. doi: 10.1128/aem.57.11.3144-3149.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idaka E., Ogawa T., Horitsu H. Oxidative pathway after reduction of p-aminoazobenzene by Pseudomonas cepacia. Bull Environ Contam Toxicol. 1987 Jul;39(1):108–113. doi: 10.1007/BF01691797. [DOI] [PubMed] [Google Scholar]
- Idaka E., Ogawa T., Horitsu H. Reductive metabolism of aminoazobenzenes by Pseudomonas cepacia. Bull Environ Contam Toxicol. 1987 Jul;39(1):100–107. doi: 10.1007/BF01691796. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Lewis B. How to develop and use a patient questionnaire. Physicians Manage. 1986 Feb;26(2):161-6, 171, 174. [PubMed] [Google Scholar]
- Millis C. D., Cai D. Y., Stankovich M. T., Tien M. Oxidation-reduction potentials and ionization states of extracellular peroxidases from the lignin-degrading fungus Phanerochaete chrysosporium. Biochemistry. 1989 Oct 17;28(21):8484–8489. doi: 10.1021/bi00447a032. [DOI] [PubMed] [Google Scholar]
- Mliki A., Zimmermann W. Purification and characterization of an intracellular peroxidase from Streptomyces cyaneus. Appl Environ Microbiol. 1992 Mar;58(3):916–919. doi: 10.1128/aem.58.3.916-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasti M. B., Pometto A. L., 3rd, Nuti M. P., Crawford D. L. Lignin-solubilizing ability of actinomycetes isolated from termite (Termitidae) gut. Appl Environ Microbiol. 1990 Jul;56(7):2213–2218. doi: 10.1128/aem.56.7.2213-2218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszczynski A., Crawford R. L. Degradation of azo compounds by ligninase from Phanerochaete chrysosporium: involvement of veratryl alcohol. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1056–1063. doi: 10.1016/0006-291x(91)90999-n. [DOI] [PubMed] [Google Scholar]
- Rafii F., Franklin W., Cerniglia C. E. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol. 1990 Jul;56(7):2146–2151. doi: 10.1128/aem.56.7.2146-2151.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra M., Crawford D. L., Hertel G. Characterization of an extracellular lignin peroxidase of the lignocellulolytic actinomycete Streptomyces viridosporus. Appl Environ Microbiol. 1988 Dec;54(12):3057–3063. doi: 10.1128/aem.54.12.3057-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra M., Crawford D. L., Pometto A. L. Extracellular Enzyme Activities during Lignocellulose Degradation by Streptomyces spp.: A Comparative Study of Wild-Type and Genetically Manipulated Strains. Appl Environ Microbiol. 1987 Dec;53(12):2754–2760. doi: 10.1128/aem.53.12.2754-2760.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxon J. J., Ryan A. J., Wright S. E. Reduction of tartrazine by a Proteus species isolated from rats. Food Cosmet Toxicol. 1966 Aug;4(4):419–426. doi: 10.1016/s0015-6264(66)80583-7. [DOI] [PubMed] [Google Scholar]
- Scheline R. R., Nygaard R. T., Longberg B. Enzymatic reduction of the azo dye, acid yellow, by extracts of Streptococcus faecalis isolated from rat intestine. Food Cosmet Toxicol. 1970 Feb;8(1):55–58. doi: 10.1016/s0015-6264(70)80223-1. [DOI] [PubMed] [Google Scholar]
- Walker R. The metabolism of azo compounds: a review of the literature. Food Cosmet Toxicol. 1970 Dec;8(6):659–676. doi: 10.1016/s0015-6264(70)80455-2. [DOI] [PubMed] [Google Scholar]
- Williams S. T., Goodfellow M., Wellington E. M., Vickers J. C., Alderson G., Sneath P. H., Sackin M. J., Mortimer A. M. A probability matrix for identification of some Streptomycetes. J Gen Microbiol. 1983 Jun;129(6):1815–1830. doi: 10.1099/00221287-129-6-1815. [DOI] [PubMed] [Google Scholar]
- Zimmermann T., Gasser F., Kulla H. G., Leisinger T. Comparison of two bacterial azoreductases acquired during adaptation to growth on azo dyes. Arch Microbiol. 1984 May;138(1):37–43. doi: 10.1007/BF00425404. [DOI] [PubMed] [Google Scholar]
- Zimmermann T., Kulla H. G., Leisinger T. Properties of purified Orange II azoreductase, the enzyme initiating azo dye degradation by Pseudomonas KF46. Eur J Biochem. 1982 Dec;129(1):197–203. doi: 10.1111/j.1432-1033.1982.tb07040.x. [DOI] [PubMed] [Google Scholar]



