Abstract
A province-wide cross-sectional seroprevalence and agroecological risk factor study of Mycobacterium avium subspecies paratuberculosis (MAP) and Neospora caninum (NC) infection among cattle in 100 cow-calf herds in Alberta was conducted. The seroprevalence of MAP in adult cattle was 1.5% across all herds. Using a widely accepted herd test cutpoint of 2 or more seropositive cows out of 30 animals tested, 7.9% of herds were estimated to be infected (95% confidence interval (CI): 2.3–23.4%). Seroprevalence of MAP differed by agroecological region; specifically, cattle and herds in areas with high soil pH (> 7.0), southern latitudes, and arid climates had a moderately reduced risk of infection (P < 0.10). Seroprevalence of NC infection was 9.7% among adult beef cattle province-wide — these levels also varied by agroecological region — with 91.0% of herds infected overall.
Résumé
Séroprévalence d’un facteur de risque agroécologique à Mycobacterium avium sous-espèce paratuberculosis et infection à Néospora caninum parmi les bovins de boucheries adultes de troupeaux vaches-veaux en Alberta, Canada. Une étude transversale de séroprévalence et de risque agroécologique à Mycobacterium avium sous-espèce paratuberculosis (MAP) et d’infection à neospora caninum (NE) parmi les bovins de 100 troupeaux vaches-veaux a été menée à travers toute la province de l’Alberta. La séroprévalence de MAP chez les bovins adultes était de 1,5 % parmi tous les troupeaux. En utilisant pour les troupeaux un seuil de décision largement accepté de 2 vaches séropositives ou plus par 30 animaux testés, 7,9 % des troupeaux étaient considérés infectés (95 %, intervalle de confiance (IC): 2,3–23,4 %). La séroprévalence du MAP variait par région agroécologique : les bovins et les troupeaux dans les régions dont le sol avait un pH élevé (> 7,0), les latitudes plus basses et les climats arides présentaient un risque d’infection moyennement réduit (P < 0,10). La séroprévalence d’infection à NC était de 9,7 % parmi les bovins adultes de l’ensemble de la province et ces niveaux variaient également selon les régions agroécologiques avec globalement 91 % des troupeaux infectés.
(Traduit par Docteur André Blouin)
Introduction
While much of the present-day interest and research emphasis pertaining to infectious diseases of livestock has shifted to pathogens with the potential to either impact international trade or cause human disease, there remains much to be learned about those diseases whose manifestations largely affect farm productivity and profitability through clinical morbidity and mortality. Often, these infections contribute to additional losses through the subclinical manifestations of culling, reproductive failure, and decreased production. Two such diseases in beef cattle are Johne’s disease (JD) and neosporosis.
Johne’s disease is a chronic infectious gastrointestinal disease of ruminants with worldwide distribution caused by the bacterium Mycobacterium avium subspecies paratuberculosis (MAP) (1). While becoming more commonly recognized as a significant cause of clinical and subclinical losses in dairy cattle operations (2,3), its importance to the beef industry has only recently been investigated in a systematic and widespread manner (4,5). Johne’s disease (paratuberculosis) is listed by the World Organization for Animal Health (OIE) (6) as a ruminant disease of concern, and the causative agent is under considerable scrutiny as a potential zoonosis (7,8). It presently represents a disease with potential for substantial economic burdens to both beef cow-calf and dairy cattle operations in Canada.
Clinically, JD appears sporadically in both beef and dairy herds, presumptively diagnosed on the appearance of unthrifty adult cattle with weight loss, chronic diarrhea, and ultimately death (9). Subclinically, it can manifest as decreased production (milk), increased culling (lost genetic potential and productivity), and decreased reproductive performance (3). Many MAP-infected dairy cattle will never progress to the clinical stages due to heavy culling pressures (10). On the other hand, MAP-infected beef cattle — usually subjected to less culling pressure so long as they produce a calf every year — may be more prone to eventually appear as a clinical case. Diagnosis of subclinical infection with MAP in ruminant species remains one of the greatest challenges to JD control, both at the individual-animal (11,12) and herd-level (13).
There have been several recent research publications concerning the seroprevalence of production-limiting infectious diseases in Canadian dairy cattle and herds (14–16), but relatively few in the peer-reviewed literature pertaining to MAP infection among Canadian beef cattle in cow-calf herds. Initial prevalence studies in Alberta (17) have suggested that levels of MAP infection in beef herds are quite low, likely similar to other jurisdictions in Canada (18), and mostly — though not always — lower than those found in the United States (4,5,19–21). Studies in dairy cattle have pointed to soil type and farm management as being associated with increased prevalence in the United States (22–24), although these same environmental risk factors have not been examined to any degree in Canadian settings, other than in a recently completed study in dairy cattle (16). Widely recognized farm management risk factors usually implicate biosecurity (purchase of infected animals), calving area and colostrum/milk management, and contamination of the feedstuffs of young stock as having large impacts on disease prevalence (1).
Neosporosis is an infectious reproductive disease caused by Neospora caninum (NC), resulting in abortion or embryonic death in infected cattle (25,26). The disease is worldwide in distribution and is most commonly acquired via point-source exposures involving domestic and wild canids (27,28). Although the disease is more commonly recognized in dairy cattle (29,30), NC has also been linked to abortions in beef herds (31–33). It has been suggested that NC might also be a secondary infection and not the only cause of abortion in infected cattle (26). An abortion attributed to neosporosis can be identified by testing the serum of the cow that aborted. Serological tests are most commonly used to diagnose infection in adult cattle (34). If antibodies to NC are not present, then it can be assumed that neosporosis did not cause the abortion (25). Although neosporosis is suspected to cause decreased milk production in infected cattle, some authors have suggested that it is abortion status, not infection, that is to blame for production losses (35,36). Neosporosis also has been linked to decreases in average daily gains among beef feedlot cattle (37). It has been noted that in the year following a neosporosis outbreak, animals that were infected during the outbreak are less likely to become pregnant the following breeding season, and typically have a higher abortion rate in the future (33). Possible risk factors for NC infection include food type, importation of replacement cows, and the presence of dogs and other wild canids on cattle farms (27). A recent study of northern Alberta beef cattle found the seroprevalence to be 9.0%, compared with 13.5% of 260 serum samples collected earlier in the 1980s (38). Few other beef studies exist in Canada, though there are a number both in the United States (32,39) and elsewhere (40) from which some relevant information can be inferred. Haddad et al (30) published a review article in 2005 that has shed light on the paucity of peer-reviewed publications concerning NC prevalence and risk factors in beef cattle in Canada.
The objectives of the present study were as follows: 1) to estimate the individual- and herd-level seroprevalence of MAP and NC among adult beef cattle and cow-calf herds, and 2) to examine some agroecological factors for their association with MAP and NC seroprevalence among adult beef cattle and cow-calf herds in Alberta. A secondary analysis was performed to assess the odds of pregnancy at time of testing, relative to MAP and NC serological status.
Materials and methods
The sampling methods, laboratory assays, and statistical analyses employed in this study closely parallel those of a similar study conducted on adult dairy cattle and herds in Alberta during 2002–2003 (16). The methods will therefore be summarized briefly here, with important differences noted where appropriate.
Study populations and herd/animal sampling procedures
As per the sampling definitions of Dohoo et al (41), target populations in this study were the adult cattle and beef cow-calf herds in Alberta. The study population included only those cattle in herds serviced by Johne’s control program accredited veterinarians in Alberta. The sampling frame was limited to the cattle owned by the client base of those accredited veterinarians. A stratified, 2-stage random sampling procedure was used to estimate individual- and herd-level seroprevalence for MAP and NC in adult beef cattle and herds in Alberta. Sampling was first stratified based on veterinarians. These veterinarians subsequently randomly selected herds and collected random samples within herds, based on randomly generated numbers supplied by the researchers.
Eligibility was limited to those veterinarians accredited by the Alberta Johne’s Control Program as of July 2002. The eligibility list included 102 cattle veterinarians working throughout Alberta. Letters soliciting participation were sent to all eligible veterinarians; of these, 60 expressed an interest, indicating that they had eligible beef cow-calf clients, while 41 actually participated, having completed the enrollment forms contained in a subsequent mail-out. Each participating veterinarian supplied the researchers with the number of beef cow-calf herds in his or her practice that contained a minimum of 30 adult cattle. Based on the data provided by the veterinarians, 2989 beef cow-calf herds (430 purebred and 2559 commercial) were potentially eligible to be selected into the study.
The number of herds to be sampled per veterinarian was randomly selected by the researchers by using probability proportional to size (of practice), with the exception that each veterinarian would be allowed to sample at least 1 herd. As a result, each of the veterinarians was asked to sample as few as 1 or as many as 6 beef herds. Each participating veterinarian was provided with an extensive 24-page information package that included random herd and cattle selection procedures. The package also included checklists, submission forms, and random number lists ranging from 1 through the number of herds indicated by the veterinarian as being eligible for inclusion in the study (a copy of the mail-out package is available from the authors on request). The veterinarian coded the herd identity to protect client confidentiality. All research communication (including results) with the herd owner was routed through the accredited veterinarian, so the names of the study participants remain unknown to the researchers. Veterinarians were remunerated with a $400.00 payment per herd for participating in the study, for selecting the herds and cattle, for collecting the survey data and serum samples, and for communicating results with the herd owner.
The study ran from October 2002 through January 2003. Minimum inclusion criteria were as follows: herds must have had at least 30 adults (females ≥ 2nd lactation, and any males ≥ 3 y). First-calf heifers and ≤ 2-year-old bulls were excluded from this count. Herds were randomly selected from client lists within clinics, and approached in order by the veterinarian using carefully specified criteria and a random-number list provided by the researchers. If a herd owner did not wish to participate, the veterinarian moved to the next herd until his or her quota was filled. Within each herd, 30 animals were randomly chosen from the eligible animals. Since most animals were tested during the autumn cattle run of 2002, veterinarians utilized a systematic random sampling scheme (42), provided by the researchers, that was appropriate to the herd size. This involved establishing a sampling interval (number of eligible adults in herd (N) divided by sample size [n = 30] = j [rounded down]) and selecting the 1st animal from among the 1st j animals by using a random number table provided by the researchers. Thereafter, at every j interval of animals through the chute, an adult cow or bull was selected until a sample size of 30 was achieved. For example, if the herd size of eligible animals (N) was 110, and n was specified as 30, j would round to every 3rd animal. If the random number was 2 (chosen between 1 and 3), then every 3rd animal starting with the 2nd and ending with the 91st animal would be selected.
Several days before a scheduled herd test, a package containing sample instructions and supplies, survey and data sheets, and shipping materials was sent from the researchers to the veterinarian. Blood samples (5–8 mL/vial) were collected into serum-separator tubes by the veterinarian from the median caudal vein (tail vein) of each selected animal (3 vials for each adult). Animal identification was recorded on each vial and on the submission form, along with age (mo), sex, dominant breed, and pregnancy status (where tested; pregnancy diagnosis was entirely voluntary and paid for by the client) at the time of testing. Samples were collected and submitted on ice (4°C) to the Alberta Agriculture, Food and Rural Development — Food Safety Division (AAFRD-FSD) Edmonton laboratories within 24 h after sampling.
Laboratory procedures and assays
Blood samples were centrifuged to serum either by the veterinarian or upon arrival at the laboratory. Serum samples from individual test animals were assigned a unique bar code number for tracking purposes and stored frozen at −72°C until use. One vial of adult serum was reserved for confirmatory testing; 2 vials were each tested for MAP and NC.
Briefly, an enzyme-linked immunosorbent assay (ELISA) (BIOCOR® Parachek® ELISA; BIOCOR Animal Health, Omaha, Nebraska, USA) was used to analyze the samples for antibody to MAP. The procedure was automated with a robot. The test sample result was determined by using the optical density (OD) and the cutoff value (the mean of the negative control plus 0.100). A positive result was an OD value greater than the cutoff value. The sensitivity and specificity of this test were estimated from previous reports at 47.3% and 99%, respectively (43).
The presence of antibodies to Neospora caninum was determined by ELISA (IDEXX® Herdchek® ELISA kit; IDEXX Laboratories, Westbrook, Maine, USA). This procedure also was automated with a robot. Serum to positive ratios (S/P) were used to determine serological status. If the S/P ratio was ≥ 0.4, the sample was classified as positive for antibodies to NC. The 0.4 S/P test cutpoint was validated in the laboratory with sensitivity estimated at 97.6% and specificity at 99.5% (44). The manufacturer’s packet insert suggests a cutpoint S/P of 0.5, which provides a slightly higher specificity, with a concomitant decline in sensitivity.
Agroecological risk factors
Legal land locations — as provided by the beef cow-calf producers — were converted to latitude and longitude with projection based on the North American datum of 1983. These were considered to be for the major “home quarter” representing the location of the calving pens. These coordinates were used to relate the individual adult cattle and herd seroprevalence data to: 1) agroecological regions (montane, boreal forest, parkland, grassland); 2) agroclimatic features (heat and soil moisture cropping limitations); 3) landscape features (plains, valleys, uplands); and 4) soil features (soil orders, texture, pH), described in detail in the Agroecological Resource Areas (ARAs) of the National Soil Database (NSDB) (45), the Agroclimatic Atlas of Alberta (46), and the Agricultural Region of Alberta Soil Inventory Database (AGRASID) (47), respectively. The agroecological regions we utilized were aggregates of those provided in the ARA database, using methods described elsewhere (48). Heat and soil moisture limitations were indices that were derived from estimated cropping limitations throughout the province (46,47). Algorithms previously described (47) were used to join and relate the geospatial soils data to the beef herd databases by using a geographical information system (GIS) (ArcMap 8.1, ESRI, Redlands, California, USA). A single soil feature representing the dominant soil class features for the upper horizon in agricultural soils (as opposed to natural or uncultivated soils) was applied to the herd database.
Statistical analyses
Statistical analyses were performed by using 2 standard statistical software packages (SPSS® version 12.0; SPSS, Chicago, Illinois, USA and STATA® release 9.2; Stata Corporation, College Station, Texas, USA).
Descriptive statistics were computed for seroprevalence data, accounting for the stratified 2-stage sampling scheme. This required that prevalence estimates and standard errors be adjusted for each of 1) the stratum (veterinarian); 2) the primary sampling unit (herd); and 3) the sampling weights (calculated as the inverse of the product of the probability of herd and adult animal [within-herd] being sampled). Individual- and herd-level seroprevalence for infection with each of MAP and NC were thereafter estimated (49). An estimate of the true individual-level prevalence was calculated as previously described (42) for MAP because of the imperfect sensitivity and specificity of the serological test.
Data on the serological status for each of MAP and NC were cross-classified with individual factors such as age, dominant breed, and pregnancy status (if tested), while being stratified by agrocecological region. Pregnancy status was not considered to be a “risk factor” per se; rather, as a cross-sectional finding at the time of sampling. Because the various agroecological factors and indices were considered likely to be “nested” within the ecoregions (potentially leading to “competing” models), these herd-level factors were cross-classified with the aggregated agroecological regions, and bivariate associations were assessed via Pearson’s chi-square test.
The association of each of the agroecological features or indices with each of individual- and herd-level serological statuses for MAP and NC was assessed first in bivariable and then in multivariable models. Ratio statistics describing the odds of seropositivity (individual or herd) for each level of the agroecological risk factors, versus a baseline level, were first estimated in a bivariable logistic regression model. Multivariable model-building approaches that incorporated potential confounders were as follows: herd size (dichotomized at the median = 117 animals) was forced into each model as a “surrogate” for unmeasured management factors. For individual-level models only, age (categorized in 3, 4, 5, and 6+ y) and dominant breed were also forced into the models. Dominant breed class was listed as “other” for breeds with less than 100 animals represented in the data set. Records with missing values for dominant breed were retained and classified as “missing breed.” Fully saturated models were created by using all agroecological variables significant (P < 0.05) in bivariable analyses. Agroecological variables were then removed in a backwards-elimination strategy. Groups of indicator variables were assessed as to significance by using the likelihood ratio χ2 statistic at P < 0.05 for significance.
A secondary analysis assessed the odds of being pregnant at the time of testing relative to each of the NC and MAP serological statuses of the adult female animal. Records with missing values for pregnancy were excluded from this analysis; this approach did not include control for confounders, as it was exploratory in nature.
Since the individual-level binary outcome responses were grouped within herds, a mixed logistic model (Stata release 9.2, XTLOGIT procedure; Stata Corp.) with a random-effect for herd (50) was used to account for this clustering. Variance components attributed to herd were estimated for all final models. This approach was not used in the herd-level analyses, where the response variable was binary with all risk factors measured at the herd level. For herd-level models, a generalized-linear-model (GLM) (51) framework (SAS7 PROC GENMOD; SAS Institute), using a binomial error distribution and a logit link function was employed. Herd-level risks for MAP infection only were modelled with positive herd (dependent variable) classification being based on 2 or more seropositive animals out of the 30 tested. For NC herd status, a single seropositive animal was sufficient for a herd to be categorized as positive.
Results
Descriptive analysis
Of the 101 herds for which samples or surveys, or both, were received, 1 herd was excluded from further analysis, because incomplete information was provided. There were a minimum of 30 and a maximum of 875 adult cattle in the beef study herds (mean = 153.5; median = 117). A total of 2996 adult cattle with complete serological data sampled from the 100 herds were retained in the analysis. Of these, all but 5 were females.
Serological evidence of infection with MAP was prevalent in 1.5% of the adult beef cattle in Alberta (95% confidence interval [CI]: 0.9–2.5 — see Table 1). Based on the imperfect performance of the serological test (47.3% sensitivity, 99% specificity), the true individual-level prevalence (42) was therefore expected to be 1.1% (95% CI: 0.0–3.2). The MAP herd-prevalence estimates (dichotomized as infected herds and noninfected herds), based on cutpoints of both 1+ and 2+ ELISA test results, are presented in Table 1. There were 28.5% of beef cow-calf herds with at least 1+ serological test result for MAP. However, because at least 1% of MAP ELISA + results in individual animals are expected to be false positives, a cutpoint of 2 or more test positive animals was used to ensure 100% herd-level specificity. The 2+ cutpoint is more widely accepted in the dairy MAP literature for sample sizes of 30 animals in herds of varying sizes (2,14), and on this basis, 7.9% of beef herds (95% CI: 2.3–23.4) in Alberta would be estimated to harbor animals infected with MAP.
Table 1.
Individual animal and herd seroprevalence for Mycobacterium avium subspecies paratuberculosis (MAP) and Neospora caninum (NC) among adult cattle (2nd calf and older cows, 3-year and older bulls) in beef cow-calf herds in Alberta
| Serologic assay | Individual (seropositive) % (95% CI) | Herd (≥ 1 seropositive) % (95% CI) | Herda (≥ 2 seropositive) % (95% CI) |
|---|---|---|---|
| MAP (n = 2996 animals in 100 herds) | 1.5 (0.9–2.5) | 28.5 (17.3–43.0) | 7.9 (2.3–23.4) |
| NC (n = 2996 animals in 100 herds) | 9.7 (7.6–12.3) | 91.0 (85.4–94.6) | — |
Herd positivity based on ≥ 2 seropositive animals applies only to the MAP-tested animals and herds
The province-wide seroprevalence of infection with NC in adult beef cattle was 9.7% (95% CI: 7.6–12.3 — see Table 1). There were 91.0% of the beef cow-calf herds in Alberta with at least a single positive animal using an ELISA S/P cutpoint of 0.4. Using the manufacturer’s recommended cutpoint of 0.5, 87.0% of herds were positive for antibodies to NC.
Infection of adult beef cattle with both NC and MAP was exceedingly rare, with only 2 jointly seropositive animals out of the 2996 tested.
Risk-factor analysis
In bivariable analyses — for both individual-level and herd-level risks for MAP and NC seropositivity — herd size was unimportant by itself (P > 0.35 for all 4 models). Data pertaining to the various individual factors, stratified by agroecological region, are cross-tabulated with cow-level serological results in Table 2.
Table 2.
Cross-tabulation of age, pregnancy status, and dominant breed by serological status for antibodies to Mycobacterium avium subspecies paratuberculosis (MAP) and Neospora caninum (NC), stratified by agroecological region (n = 2991 adult cowsa)
| Serological response
|
||||||
|---|---|---|---|---|---|---|
| MAP
|
NC
|
|||||
| Agroecological region | Factor | Level of factor | (+) | (−) | (+) | (−) |
| Montane | Age | < 48 months | 0 | 25 | 3 | 22 |
| 48 to < 60 months | 0 | 36 | 2 | 34 | ||
| 60 to < 72 months | 1 | 31 | 0 | 32 | ||
| ≥ 72 months | 0 | 87 | 7 | 80 | ||
| Pregnant | Not tested | 0 | 0 | 0 | 0 | |
| Pregnant | 1 | 163 | 11 | 153 | ||
| Open | 0 | 16 | 1 | 15 | ||
| Breed | Not recorded | 1 | 36 | 5 | 32 | |
| Angus | 0 | 50 | 2 | 48 | ||
| Charolais | 0 | 8 | 1 | 7 | ||
| Hereford | 0 | 29 | 0 | 29 | ||
| Limousin | 0 | 7 | 0 | 7 | ||
| Otherb | 0 | 5 | 0 | 5 | ||
| Red Angus | 0 | 12 | 1 | 11 | ||
| Simmental | 0 | 32 | 3 | 29 | ||
| Boreal forest | Age | < 48 months | 1 | 74 | 9 | 75 |
| 48 to < 60 months | 2 | 158 | 29 | 160 | ||
| 60 to < 72 months | 2 | 143 | 16 | 145 | ||
| ≥ 72 months | 9 | 538 | 85 | 547 | ||
| Pregnant | Not tested | 7 | 374 | 71 | 310 | |
| Pregnant | 7 | 503 | 62 | 448 | ||
| Open | 0 | 36 | 6 | 30 | ||
| Breed | Not recorded | 0 | 11 | 1 | 10 | |
| Angus | 2 | 146 | 15 | 133 | ||
| Charolais | 3 | 153 | 28 | 128 | ||
| Hereford | 1 | 126 | 10 | 117 | ||
| Limousin | 0 | 29 | 11 | 18 | ||
| Otherb | 4 | 158 | 20 | 142 | ||
| Red Angus | 1 | 25 | 6 | 20 | ||
| Simmental | 3 | 265 | 48 | 220 | ||
| Parkland | Age | < 48 months | 1 | 265 | 26 | 266 |
| 48 to < 60 months | 2 | 196 | 14 | 198 | ||
| 60 to < 72 months | 3 | 198 | 20 | 201 | ||
| ≥ 72 months | 9 | 610 | 61 | 619 | ||
| Pregnant | Not tested | 0 | 243 | 26 | 217 | |
| Pregnant | 14 | 951 | 84 | 881 | ||
| Open | 1 | 75 | 11 | 65 | ||
| Breed | Not recorded | 2 | 60 | 10 | 52 | |
| Angus | 1 | 245 | 18 | 228 | ||
| Charolais | 4 | 270 | 27 | 247 | ||
| Hereford | 1 | 81 | 6 | 76 | ||
| Limousin | 1 | 66 | 5 | 62 | ||
| Otherb | 1 | 94 | 10 | 85 | ||
| Red Angus | 1 | 94 | 3 | 92 | ||
| Simmental | 4 | 359 | 42 | 321 | ||
| Grassland | Age | < 48 months | 0 | 36 | 2 | 36 |
| 48 to < 60 months | 0 | 103 | 4 | 103 | ||
| 60 to < 72 months | 0 | 73 | 4 | 73 | ||
| ≥ 72 months | 1 | 388 | 17 | 388 | ||
| Pregnant | Not tested | 0 | 90 | 2 | 88 | |
| Pregnant | 1 | 468 | 20 | 449 | ||
| Open | 0 | 41 | 5 | 36 | ||
| Breed | Not recorded | 0 | 30 | 4 | 26 | |
| Angus | 0 | 107 | 3 | 104 | ||
| Charolais | 1 | 95 | 7 | 89 | ||
| Hereford | 0 | 156 | 3 | 153 | ||
| Limousin | 0 | 25 | 0 | 25 | ||
| Otherb | 0 | 38 | 4 | 34 | ||
| Red Angus | 0 | 117 | 3 | 114 | ||
| Simmental | 0 | 31 | 3 | 28 | ||
Bulls were excluded from this table; incomplete information was available on 2 cows
Other recognized breeds comprised those representing less than 100 cows: Beef Booster (n = 37), Blonde d’Aquitane (n = 4), Chianina (n = 1), Gelbvieh (n = 49), Holstein (n = 3), Jersey (n = 4), Longhorn (n = 1), Maine Anjou (n = 18), Murray Grey (n = 57), Pinzgauer (n = 1), Saler (n = 72), Shorthorn (n = 31), and Tarantais (n = 22)
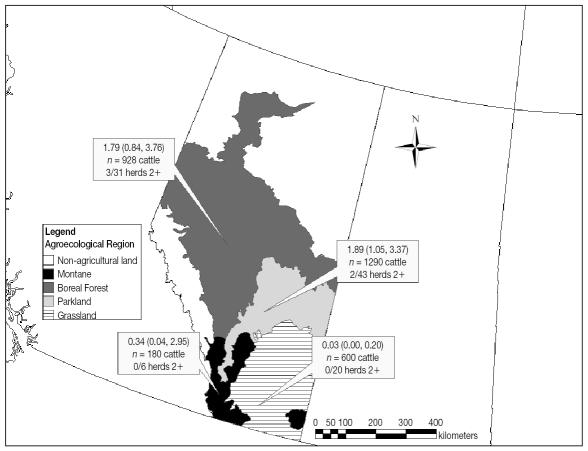
The seroprevalence of MAP infection in both adult beef cattle and herds is mapped by agroecological region in Figure 1. For reference, the agroclimatic and soil features at each of the 100 farm locations are cross-tabulated by agroecological region in Table 3. In the grassland and montane regions of Alberta, there were no herds that met the 2+ criterion to be classified as MAP positive, based on serological testing. The survey design-adjusted (49) estimate of individual-level seroprevalence of MAP infection was very low in the grassland region at 0.03%, while it was highest in the parkland region at 1.89%.
Figure 1.
Mycobacterium avium subspecies paratuberculosis (MAP) seroprevalence among adult beef cattle in cow-calf herds in Alberta. Cow-calf herds were classified as seropositive based on at least 2 positive ELISAs for MAP infection.
Table 3.
Contingency table illustrating association of ecological risk factors with the aggregated index of agroecological region at the herd-level (n = 100 cow-calf herds)
| Agroecological region
|
||||||
|---|---|---|---|---|---|---|
| Ecological risk factor | Level of risk factor | Grassland | Montane | Parkland | Boreal forest | Significance (P-value)a |
| Soil pH | <7.0 | 7 | 2 | 39 | 31 | < 0.001 |
| ≤7.0 | 13 | 4 | 4 | 0 | ||
| Arid climate (soil moisture limited) | No | 0 | 0 | 21 | 31 | < 0.001 |
| Yes | 20 | 6 | 22 | 0 | ||
| Salt in soil | No | 12 | 6 | 35 | 31 | 0.001 |
| Yes | 8 | 0 | 8 | 0 | ||
| Heat limitation | Area 2 (low) | 14 | 6 | 32 | 0 | < 0.001 |
| for cropping | Areas 3, 4, and 5 (high) | 6 | 0 | 11 | 31 | |
P-values were derived from asymptotic Pearson chi-square 2-sided tests of association comparing agroecological region to each of the other ecological risk factors
The agroecological region was the only ecological risk factor shown to be significantly associated (P = 0.044) with the risk of MAP seropositivity at the individual level (Table 4, Model I). In the final model, the variance component contributed by the herd effect was 11.4%. The grassland and montane regions (southern areas of Alberta with higher soil pH, low soil moisture, and less heat limitations) presented a lower individual-level risk than did parkland and boreal forest regions. Climatic aridity, soil features (pH and salinity), and heat limitations were not significant (P > 0.05) by themselves in bivariable analyses. When measured on a continuous scale and modelled as a linear function, soil pH was not associated with MAP seroprevalence. When dichotomized at pH 7.0, those animals raised on land with basic soils were at a somewhat lowered (though still nonsignificant) risk of infection (OR = 0.40; P = 0.15). None of the other risk factors was significantly associated — either at the individual or the herd level — with MAP seropositivity. However, a moderate change in the parameter estimates for association of agroecological regions with MAP, when comparing the models with and without confounders, suggested that their forced inclusion in a multivariable model was warranted despite no apparent association with MAP serological status. Herd-level serological status (classified as containing either 1 or 2 seropositive animals out of the 30 tested) was not associated (P > 0.20) with any of the agroecological variables, either in bivariable models or when controlling for herd size.
Table 4.
Multivariable logistic regression models illustrating the association of agroecological region with the risk of seropositivity among individual beef cattle in Alberta to: Model I: antibodies to Mycobacterium avium subspecies paratuberculosis (MAP), and Model II: antibodies to Neospora caninum (NC). All models are adjusted for within-herd dependence of outcomes by including a random-effect for herd. Herd size (dichotomized at the median), age, and dominant breed were forced into the models as potential confounders.a,b Intercepts are not shown.
| Risk factor | Level of risk factor | Odds ratio (OR) | 95% confidence interval (OR) | P-valuec |
|---|---|---|---|---|
| Model I: MAP | ||||
| Herd size | < 117 adults | — | — | 0.406 |
| ≥ 117 adults | 1.42 | (0.62, 3.28) | ||
| Age | < 48 months | — | — | 0.427 |
| 48 to , 60 months | 1.59 | (0.28, 8.98) | ||
| 60 to , 72 months | 2.65 | (0.52, 13.60) | ||
| ≥ 72 months | 2.69 | (0.60, 12.05) | ||
| Dominant breed | Not recorded | — | — | 0.659 |
| Angus | 0.16 | (0.03, 1.04) | ||
| Charolais | 0.38 | (0.07, 1.92) | ||
| Hereford | 0.19 | (0.03, 1.43) | ||
| Limousin | 0.23 | (0.02, 2.78) | ||
| Otherd | 0.46 | (0.08, 2.56) | ||
| Red Angus | 0.37 | (0.05, 2.76) | ||
| Simmental | 0.27 | (0.05, 1.35) | ||
| Agroecological region | Montane | — | — | 0.044 |
| Boreal forest | 3.64 | (0.38, 35.27) | ||
| Parkland | 2.85 | (0.31, 26.66) | ||
| Grassland | 0.37 | (0.02, 6.97) | ||
| Model II: NC | ||||
| Herd size | < 117 adults | — | — | 1.000 |
| ≥ 117 adults | 1.00 | (0.66, 1.51) | ||
| Age | < 48 months | — | — | 0.560 |
| 48 to , 60 months | 0.84 | (0.52, 1.36) | ||
| 60 to , 72 months | 0.78 | (0.47, 1.28) | ||
| ≥ 72 months | 0.99 | (0.66, 1.49) | ||
| Dominant breed | Not recorded | — | — | 0.036 |
| Angus | 0.38 | (0.16, 0.92) | ||
| Charolais | 0.62 | (0.26, 1.48) | ||
| Hereford | 0.29 | (0.11, 0.77) | ||
| Limousin | 0.40 | (0.14, 1.19) | ||
| Otherd | 0.49 | (0.20, 1.23) | ||
| Red Angus | 0.35 | (0.13, 0.95) | ||
| Simmental | 0.70 | (0.30, 1.63) | ||
| Agroecological region | Montane | — | — | 0.002 |
| Boreal forest | 2.64 | (0.99, 7.05) | ||
| Parkland | 1.48 | (0.56, 3.89) | ||
| Grassland | 0.82 | (0.29, 2.37) | ||
Estimates of the odds ratios assessing the association of agroecological region with MAP changed a maximum of 35% comparing the model with confounders to the uncontrolled model
Estimates of the odds ratios assessing the association of agroecological region with NC changed a maximum of 24% comparing the model with confounders to the uncontrolled model
P-values were derived based on the likelihood ratio χ2 test for nested models with k-1 degrees of freedom (for categorical variables with k levels)
Other recognized breeds comprised those representing less than 100 cows: Beef Booster (n = 37), Blonde d’Aquitane (n = 4), Chianina (n = 1), Gelbvieh (n = 49), Holstein (n = 3), Jersey (n = 4), Longhorn (n = 1), Maine Anjou (n = 18), Murray Grey (n = 57), Pinzgauer (n = 1), Saler (n = 72), Shorthorn (n = 31), and Tarantais (n = 22)
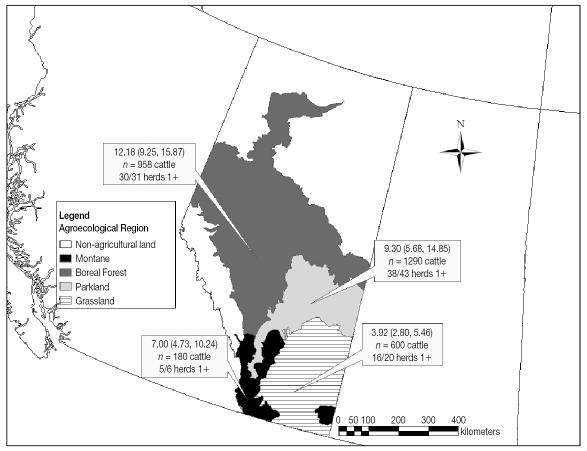
The seroprevalence of NC infection among individual beef cattle reared in the grassland agroecological region of Alberta (Figure 2) was lower (3.92%) when compared with either of the more northerly regions of parkland (9.3%) or boreal forest (12.2%). Logistic regression analysis of the individual-level risk of NC seropositivity, accounting for within-herd clustering by including a random effect for herd, confirmed a strong (P = 0.002) association with region (Table 4, Model II). The variance component contributed by herd effect was 14.2% in this model. Unlike for MAP, individual NC seropositivity was also associated in multivariable models (not shown) to be significantly associated with decreased risks in areas with basic soils (pH dichotomized at 7.0 — adjusted odds ratio [OR] = 0.36; 95% CI : 0.20, 0.65) and sparing climate features, including lessened heat limitations (low heat limitations versus highly heat limited areas — adjusted OR = 0.59; 95% CI: 0.38, 0.92) and soil aridity (highly arid versus low arid — adjusted OR = 0.486; 95% CI: 0.32, 0.74). Soil salinity was unimportant (P > 0.05). Differences in risk of NC seropositivity at the herd level (models not shown) were likewise attributable to either soil pH or aridity (but not both; P < 0.05), but not to either agroecological region (P = 0.345) or heat limitations (P = 0.145).
Figure 2.
Neospora caninum (NC) seroprevalence among adult beef cattle in cow-calf herds in Alberta. Cow-calf herds were classified as seropositive based on at least 1 positive ELISA for NC.
Pregnancy analysis
In a mixed bivariable logistic regression model, accounting for clustering of responses by herd, NC seropositivity was significantly (P = 0.049) associated with a decreased odds of being diagnosed as pregnant (OR = 0.61; 95% CI: 0.37, 1.00) at the herd testing date among the 2279 adult cows from 78 herds that were subjected to the voluntary diagnostic procedure by the herd veterinarian. A similar model, with MAP serological status as the independent variable, was nonsignificant (P = 0.556).
Discussion
Since the study population included only those cattle in herds serviced by accredited Johne’s control program veterinarians in Alberta, client-owned herds of accredited veterinarians may not be representative of the Alberta populations of herds and adult beef cattle. While this cannot be determined, several efforts were made to ensure maximum generalizability of results. As of August 2002, when study participation was being solicited, 102 veterinarians already were accredited. The initial accreditation process involved an outreach program offered by AAFRD-FSD in 6 regional areas blanketing the province during the late summer and early fall of 2001. Even if not every veterinarian became accredited, a high proportion of rural food animal practices were likely to be represented.
Because the sampling of beef herds occurred during the fall processing period, in some herds, the veterinarian was requested to also perform pregnancy diagnoses. When the procedure was performed, these data were recorded (along with age, dominant breed, and sex). Therefore, missing pregnancy status information occurred on a herd-by-herd basis. While the age and breed of the animals appeared to be reasonably representative of the eligible Alberta herd as a whole, bulls were under-represented (n = 5). This is possibly because bulls being processed through chutes would likely appear at the very end of the run. Rounding down of j (necessary to avoid undersized sampling) would result in a lower probability of the very last animals in the chute being selected. In addition, many herds were likely not including adult bulls in their fall processing schedules and these animals were simply unavailable to be sampled.
Beef cattle and beef herds are generally at lower risk of being infected with MAP than are dairy cattle (4), though this is not always the case, as overall seroprevalence among cattle may vary greatly by geographical region worldwide. However, within any given region, beef cattle tend to exhibit a lower infection risk than dairy cattle (16,19,21). A preliminary study conducted in 2000 (17) included 326 Alberta beef cow-calf operations from 30 counties; 3632 randomly selected beef cattle were tested by commercial ELISA for seroprevalence of antibodies to MAP, when it was found that the serologic prevalence of MAP infection in Alberta was 2.13%, with 20 of the 30 counties having at least 1 positive case (17). In a similar study in beef cattle conducted in Saskatchewan (18), only 15 of 1799 serum samples tested positive, giving a herd-level prevalence of 15.2%, if 1 positive cow constituted a positive herd, and 3.0%, if 2 cows made a herd positive. These results suggest that adult beef cattle in cow-calf herds in Alberta may exhibit seroprevalence to MAP similar to that of other studies conducted recently in Canada. The seroprevalence in the present study was 1.5% across all herds, with 7.9% of herds considered positive, based on 2 or more seropositive animals. Importantly, the study in Saskatchewan (18) was conducted only on cattle utilizing community pastures, whereas the present study sampled across a study population of beef cow-calf herds in Alberta, without regards to community pasture use.
In the United States, results in beef cattle herds have been variable, though usually of higher seroprevalence when compared with that of the present study. In the largest study reported, a commercial ELISA was used to determine the disease status of 10 371 cows in 380 beef herds in 21 states. A herd was classified as positive if there were 1 or more seropositive cows present, resulting in only 30 operations being classified as seropositive (4). In Missouri, a study was conducted with samples from dairy and beef herds being selected on a semirandom basis (19). A total of 1954 cattle from 89 herds were tested with 101 cattle having seropositive results (5%). Forty-two of the 89 herds were positive (47%). A study of 79 herds of beef cattle in Alabama gave 166 of 2073 (8%) animals with seropositive results for MAP (20). Fifty of the 79 herds had at least 1 seropositive cow (53.5%). Positive herds had an average of 3.3 infected cows each.
The agroecological risk factor for MAP seroprevalence identified in this study (a composite index of climate, soil features, terrain, and vegetative covering) was similar to those noted elsewhere in dairy cattle (16,22–24), though few studies exist that have examined these factors with beef cattle. Two major inhibitors to MAP survival outside the host (elevated soil pH and climatic aridity) were not by themselves significantly associated with a decreased risk of seropositivity in the present study, even though they are generally component features of those agroecological regions with decreased risk (Table 3). It should be noted that with rare seroprevalence levels, such as those found in the present study, it is less likely that multiple risk factors might explain subtle differences in seroprevalence. Although it cannot be directly inferred from the present study, the stocking density (carrying capacity per hectare of land) of cattle in the parkland and boreal forest ecoregions of Alberta is readily recognized to exceed that for the grassland and montane regions. These latter 2 regions are also the only areas of the province where producers can practice traditional ranching, as opposed to farming; that is, with less supplemental feeding, shortened winter confinement, and some year-round grazing possible. These are testable hypotheses that bear further study as to their true importance; that is, that extensive range management practices, in combination with agroecological features that may include soil pH, climatic aridity, or both, may be protective against the risk of MAP infection in beef cow-calf herds. Herd-level seropositivity did not differ significantly by agroecological region, though it must be noted that there were only 5 herds that met the criteria to be labelled as seropositive, based on a cutpoint of 2 or more test positive animals, and none of them was from the grassland or montane regions of Alberta.
In this study, seroprevalence for infection with NC was estimated to be at levels that are comparable with the seroprevalence of NC of beef cattle in other studies in Canada (38,39). Overall, the seroprevalence in Canada is also similar to the prevalence in the United States, Australia, and Great Britain (39,40,52). Almost all herds have some evidence of infection — past versus recent infection is not discernable from cross-sectional studies. Though just significant (P = 0.05), there was a decreased odds of pregnancy (adjusted OR = 0.60) for NC seropositive cows presenting as pregnant at fall testing. This finding is consistent with the findings of others (33). Serological status across all cattle was somewhat variable by herd and region. Association of agroecological region with the risk of seropositivity to NC was similar to that of MAP. It is likely that the factors that bring cattle in closer contact with the point source of infection (wild or domestic canids) would also lead to higher risks of seroprevalence. Heavily treed grazing areas, as found in boreal forest and parkland areas, provide prime habitat for wild canids and their natural prey. Ranching practices in the grassland region in particular, and montane region to a lesser degree, may provide a sparing effect, reducing exposure to NC infection. While heat limitations, soil pH, and aridity had an effect (by themselves) in multivariable individual-level analyses, it is unclear as to the biological mechanism by which the association would be causal. The agroecological findings are compatible with known biological features of the disease that include both domestic and wild canid hosts and typically a point source — rather than propagated — infection. Survival of the organism outside either secondary or primary hosts is likely of little consequence. Therefore, it seems likely that the variable seroprevalence of NC infection across agroecological regions is attributable to the variable land use and ranch management practices suited to these regions, as opposed to direct effects of the ecological regions on the infectious agent itself. Seroprevalence of NC infection in the present study was generally lower at the individual-cow level, but only moderately lower at the herd level when compared with the dairy cattle study conducted in parallel with the beef study (16). Only soil pH and aridity remained associated with herd-level risk, while aggregated ecological region and heat limitations became less important than in the individual-level models. The broad distribution of seropositive animals across many herds suggests that completely avoiding the introduction of the agent into the herd is likely to be more difficult and of less consequence than in limiting the actual number of animals within each herd that are exposed to NC.
Acknowledgments
The authors thank the beef cow-calf farmers and ranchers and their Alberta Johne’s Control Program-accredited veterinarians for their cooperation. In addition, we thank the scientists and technical staff of the Agri-food Laboratories Branch of the Food Safety Division of Alberta Agriculture, Food and Rural Development for their efforts (sample processing, MAP and NC testing). Geographical information systems (GIS) and AGRASID 3.0 Alberta soil data analysis and support were graciously provided by resource specialists from the Conservation and Development Division of AAFRD and from Agriculture and Agri-food Canada. CVJ
Footnotes
This study was supported by Alberta Agriculture, Food and Rural Development (AAFRD) and the Western Economic Partnership Agreement (WEPA).
References
- 1.Whittington RJ, Sergeant ES. Progress towards understanding the spread, detection and control of Mycobacterium avium subspecies paratuberculosis in animal populations. Aust Vet J. 2001;79:267–278. doi: 10.1111/j.1751-0813.2001.tb11980.x. [DOI] [PubMed] [Google Scholar]
- 2.National Animal Health Monitoring System. Johne’s Disease on U.S. Dairy Operations. #N245.1097. Fort Collins, Colorado: United States Department of Agriculture, Animal and Plant Health Inspection Service; Veterinary Services; Centers for Epidemiology and Animal Health, 1997.
- 3.Goodell G M, Hirst H, Garry F, Dinsmore RP. Comparison of cull rates and milk production of clinically normal dairy cows grouped by ELISA Mycobacterium avium subspecies paratuberculosis serum antibody results. Proc 9th Symp Int Soc Vet Epidemiol Econ [CD-ROM] Abstract ID 579.
- 4.Dargatz DA, Byrum BA, Hennager SG, et al. Prevalence of antibodies against Mycobacterium avium subspecies paratuberculosis among beef cow-calf herds. J Am Vet Med Assoc. 2001;219:497–501. doi: 10.2460/javma.2001.219.497. [DOI] [PubMed] [Google Scholar]
- 5.Roussel AJ, Libal MC, Whitlock RL, Hairgrove TB, Barling KS, Thompson JA. Prevalence of and risk factors for paratuberculosis in purebred beef cattle. J Am Vet Med Assoc. 2005;226:773–778. doi: 10.2460/javma.2005.226.773. [DOI] [PubMed] [Google Scholar]
- 6.OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees). Paris, France: Office International des Epizooties, 2004:347–359. [PubMed]
- 7.Hermon-Taylor J. Causation of Crohn’s disease: The impact of clusters. Gastroenterology. 1993;104:643–646. doi: 10.1016/0016-5085(93)90438-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant IR. Zoonotic potential of Mycobacterium avium ssp. paratuberculosis: The current position. J Appl Microbiol. 2005;98:1282–1293. doi: 10.1111/j.1365-2672.2005.02598.x. [DOI] [PubMed] [Google Scholar]
- 9.Stabel JR. Johne’s disease: A hidden threat. J Dairy Sci. 1998;81:283–288. doi: 10.3168/jds.S0022-0302(98)75577-8. [DOI] [PubMed] [Google Scholar]
- 10.Strickland SJ, Scott HM, Libal M, Roussel Jr A, Jordan E. Effects of seasonal climatic conditions on the diagnosis of Mycobacterium avium subspecies paratuberculosis in dairy cattle. J Dairy Sci. 2005;88:2432–2440. doi: 10.3168/jds.S0022-0302(05)72921-0. [DOI] [PubMed] [Google Scholar]
- 11.Collins MT. Diagnosis of paratuberculosis. Vet Clin North Am Food. 1996;12:357–371. doi: 10.1016/s0749-0720(15)30411-4. [DOI] [PubMed] [Google Scholar]
- 12.Manning EJ, Collins MT. Mycobacterium avium subsp. paratuberculosis: Pathogen, pathogenesis and diagnosis. Rev Sci Tech. 2001;20:133–150. doi: 10.20506/rst.20.1.1275. [DOI] [PubMed] [Google Scholar]
- 13.Jordan D. Aggregate testing for the evaluation of Johne’s disease herd status. Aust Vet J. 1996;73:16–19. doi: 10.1111/j.1751-0813.1996.tb09947.x. [DOI] [PubMed] [Google Scholar]
- 14.VanLeeuwen JA, Keefe GP, Tremblay R, Power C, Wichtel JJ. Seroprevalence of infection with Mycobacterium avium subspecies paratuberculosis, bovine leukemia virus, and bovine viral diarrhea virus in maritime Canada dairy cattle. Can Vet J. 2001;42:193–198. [PMC free article] [PubMed] [Google Scholar]
- 15.VanLeeuwen JA, Forsythe L, Tiwari A, Chartier R. Seroprevalence of antibodies against bovine leukemia virus, bovine viral diarrhea virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in dairy cattle in Saskatchewan. Can Vet J. 2005;46:56–58. [PMC free article] [PubMed] [Google Scholar]
- 16.Scott HM, Sorensen O, Wu JTY, Chow EYW, Manninen K, VanLeeuwen JA. Seroprevalence of Mycobacterium avium subspecies paratuberculosis, Neospora caninum, Bovine leukemia virus, and Bovine viral diarrhea virus infection among dairy cattle and herds in Alberta and agroecological risk factors associated with seropositivity. Can Vet J. 2006;47:981–991. [PMC free article] [PubMed] [Google Scholar]
- 17.Wu JTY, Chow EYW, Ollis G. A Survey to Estimate the Seroprevalence of Johne’s Disease in Alberta Beef Herds. Edmonton, Alberta: Government of Alberta — Agri-Food Laboratories Branch, Food Safety Division, AAFRD, 2001.
- 18.Waldner CL, Cunningham GL, Janzen ED, Campbell JR. Survey of Mycobacterium avium subspecies paratuberculosis serological status in beef herds on community pastures in Saskatchewan. Can Vet J. 2002;43:542–546. [PMC free article] [PubMed] [Google Scholar]
- 19.Thorne JG, Hardin LE. Estimated prevalence of paratuberculosis in Missouri, USA cattle. Prev Vet Med. 1997;31:51–57. doi: 10.1016/s0167-5877(96)01138-5. [DOI] [PubMed] [Google Scholar]
- 20.Hill BB, West M, Brock KV. An estimated prevalence of Johne’s disease in a subpopulation of Alabama beef cattle. J Vet Diagn Invest. 2003;15:21–25. doi: 10.1177/104063870301500105. [DOI] [PubMed] [Google Scholar]
- 21.Pence M, Baldwin C, Black CC., III The seroprevalence of Johne’s disease in Georgia beef and dairy cull cattle. J Vet Diagn Invest. 2003;15:475–477. doi: 10.1177/104063870301500514. [DOI] [PubMed] [Google Scholar]
- 22.Johnson-Ifearulundu YJ, Kaneene JB. Relationship between soil type and Mycobacterium paratuberculosis. J Am Vet Med Assoc. 1997;210:1735–1740. [PubMed] [Google Scholar]
- 23.Johnson-Ifearulundu Y, Kaneene JB. Distribution and environmental risk factors for paratuberculosis in dairy cattle herds in Michigan. Am J Vet Res. 1999;60:589–596. [PubMed] [Google Scholar]
- 24.Ward MP, Perez AM. Association between soil type and paratuberculosis in cattle herds. Am J Vet Res. 2004;65:10–14. doi: 10.2460/ajvr.2004.65.10. [DOI] [PubMed] [Google Scholar]
- 25.Dubey JP. Neosporosis in cattle: Biology and economic impact. J Am Vet Med Assoc. 1999;214:1160–1163. [PubMed] [Google Scholar]
- 26.Campbell RS. Neosporosis of cattle. Vet Rec. 2001;148:188. [PubMed] [Google Scholar]
- 27.Wouda W. Diagnosis and epidemiology of bovine neosporosis: A review. Vet Q. 2000;22:71–74. doi: 10.1080/01652176.2000.9695028. [DOI] [PubMed] [Google Scholar]
- 28.Dijkstra T, Barkema HW, Eysker M, Hesselink JW, Wouda W. Natural transmission routes of Neospora caninum between farm dogs and cattle. Vet Parasitol. 2002;105:99–104. doi: 10.1016/s0304-4017(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 29.Keefe GP, VanLeeuwen JA. Neospora then and now: Prevalence of Neospora caninum in Maritime Canada in 1979, 1989, and 1998. Can Vet J. 2000;41:864–866. [PMC free article] [PubMed] [Google Scholar]
- 30.Haddad JPA, Dohoo IR, VanLeeuwen JA. A review of Neospora caninum in dairy and beef cattle — A Canadian perspective. Can Vet J. 2005;46:230–243. [PMC free article] [PubMed] [Google Scholar]
- 31.Waldner CL, Janzen ED, Henderson J, Haines DM. Outbreak of abortion associated with Neospora caninum infection in a beef herd. J Am Vet Med Assoc. 1999;215:1485–1489. [PubMed] [Google Scholar]
- 32.Sanderson MW, Gay JM, Baszler TV. Neospora caninum seroprevalence and associated risk factors in beef cattle in the northwestern United States. Vet Parasitol. 2000;90:15–24. doi: 10.1016/s0304-4017(00)00234-x. [DOI] [PubMed] [Google Scholar]
- 33.Waldner CL, Henderson J, Wu JT, Breker K, Chow EY. Reproductive performance of a cow-calf herd following a Neospora caninum-associated abortion epidemic. Can Vet J. 2001;42:355–360. [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson R, Harper PA, Reichel MP, Ellis JT. Progress in the sero-diagnosis of Neospora caninum infections of cattle. Parasitol Today. 2000;16:110–114. doi: 10.1016/s0169-4758(99)01604-x. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez J, Risco C, Donovan A. Association between exposure to Neospora caninum and milk production in dairy cows. J Am Vet Med Assoc. 2001;219:632–635. doi: 10.2460/javma.2001.219.632. [DOI] [PubMed] [Google Scholar]
- 36.Hobson JC, Duffield TF, Kelton D, et al. Neospora caninum serostatus and milk production of Holstein cattle. J Am Vet Med Assoc. 2002;221:1160–1164. doi: 10.2460/javma.2002.221.1160. [DOI] [PubMed] [Google Scholar]
- 37.Barling KS, Lunt DK, Snowden KF, Thompson JA. Association of serologic status for Neospora caninum and postweaning feed efficiency in beef steers. J Am Vet Med Assoc. 2001;219:1259–62. doi: 10.2460/javma.2001.219.1259. [DOI] [PubMed] [Google Scholar]
- 38.Waldner CL, Henderson J, Wu JT, Coupland R, Chow EY. Seroprevalence of Neospora caninum in beef cattle in northern Alberta. Can Vet J. 2001;42:130–132. [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez I, Choromanski L, Rodgers SJ, Weinstock D. Survey of Neospora caninum antibodies in dairy and beef cattle from five regions of the United States. Vet Ther. 2002;3:396–401. [PubMed] [Google Scholar]
- 40.Stoessel Z, Taylor LF, McGowan MR, Coleman GT, Landmann JK. Prevalence of antibodies to Neospora caninum within central Queensland beef cattle. Aust Vet J. 2003;81:165–166. doi: 10.1111/j.1751-0813.2003.tb11081.x. [DOI] [PubMed] [Google Scholar]
- 41.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. Charlottetown, Prince Edward Island: AVC Inc., 2003.
- 42.Martin SW, Meek AH, Willeberg P. Veterinary Epidemiology: Principles and Methods. Ames, Iowa: Iowa State Univ Pr, 1987.
- 43.Collins MT, Sockett DC, Ridge S, Cox JC. Evaluation of a commercial enzyme-linked immunosorbent assay for Johne’s disease. J Clin Microbiol. 1991;29:272–276. doi: 10.1128/jcm.29.2.272-276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu JTY, Dreger S, Chow EYW, Bowlby EE. Validation of 2 commercial Neospora caninum antibody enzyme linked immunosorbent assays. Can J Vet Res. 2002;66:264–271. [PMC free article] [PubMed] [Google Scholar]
- 45.The National Land and Water Information Service: Agriculture and Agri-food Canada [database on the internet] Agroecological Resource Area Databases for the Prairies. Available from http://sis.agr.gc.ca/cansis/nsdb/ara/intro.html Last accessed 17 July 2006.
- 46.Alberta Agriculture, Food and Rural Development: Government of Alberta [homepage on the internet] c2003–2006 Agroclimatic Atlas of Alberta: Introduction. Available from http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/sag6278 Last accessed 17 July 2006.
- 47.Alberta Agriculture, Food and Rural Development: Government of Alberta [database on the internet] c2003–2006 Agricultural Region of Alberta Soil Inventory Database. Available from http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/sag3249?opendocument Last accessed 17 July 2006.
- 48.Scott HM, Soskolne CL, Martin SW, et al. Lack of associations between air emissions from sour-gas processing plants and indices of beef cow-calf herd health and productivity in Alberta, Canada. Prev Vet Med. 2003;57:35–68. doi: 10.1016/s0167-5877(02)00206-4. [DOI] [PubMed] [Google Scholar]
- 49.StataCorp. Stata Survey Data Reference Manual: Release 9. College Station, Texas: Stata Corporation, 2005:118–210.
- 50.StataCorp. Stata Longitudinal/Panel Data: Release 9. College Station, Texas: Stata Corporation, 2005:161–176.
- 51.McCullagh P, Nelder JA. Generalized Linear Models. Boca Raton, Florida: Chapman & Hall/CRC, 1989.
- 52.Davison HC, French NP, Trees AJ. Herd-specific and age-specific seroprevalence of Neospora caninum in 14 British dairy herds. Vet Rec. 1999;144:547–550. doi: 10.1136/vr.144.20.547. [DOI] [PubMed] [Google Scholar]