Abstract
Background
Only limited data exist on lactation as an exposure source of persistent perfluorinated chemicals (PFCs) for children.
Objectives
We studied occurrence and levels of PFCs in human milk in relation to maternal serum together with the temporal trend in milk levels between 1996 and 2004 in Sweden. Matched, individual human milk and serum samples from 12 primiparous women in Sweden were analyzed together with composite milk samples (25–90 women/year) from 1996 to 2004.
Results
Eight PFCs were detected in the serum samples, and five of them were also above the detection limits in the milk samples. Perfluorooctanesulfonate (PFOS) and perfluorohexanesulfonate (PFHxS) were detected in all milk samples at mean concentrations of 0.201 ng/mL and 0.085 ng/mL, respectively. Perfluorooctanesulfonamide (PFOSA), perfluorooctanoic acid (PFOA), and perfluorononanoic acid (PFNA) were detected less frequently.
Discussion
The total PFC concentration in maternal serum was 32 ng/mL, and the corresponding milk concentration was 0.34 ng/mL. The PFOS milk level was on average 1% of the corresponding serum level. There was a strong association between increasing serum concentration and increasing milk concentration for PFOS (r2 = 0.7) and PFHxS (r2 = 0.8). PFOS and PFHxS levels in composite milk samples were relatively unchanged between 1996 and 2004, with a total variation of 20 and 32% coefficient of variation, respectively.
Conclusion
The calculated total amount of PFCs transferred by lactation to a breast-fed infant in this study was approximately 200 ng/day. Lactation is a considerable source of exposure for infants, and reference concentrations for hazard assessments are needed.
Keywords: human exposure, LC-MS, PFC, PFOA, PFOS
An increasing number of studies show that humans are exposed to a large number of perfluorinated chemicals (PFCs), including perfluorooctane sulfonate (PFOS) (Calafat et al. 2006b; Karrman et al. 2006b; Yeung et al. 2006). Pooled serum from Australians < 16 years of age (median age 11–13 years) had equal or higher levels of several PFCs compared with adults (Karrman et al. 2006a). PFOS concentration in 2- to 12-year-old children in the United States ranged between 6.7 and 515 ng/mL, and the mean concentration of PFOS and additional PFCs exceeded those for adult and elderly populations (Olsen et al. 2004). Exposure routes for children therefore need to be assessed.
The surfactant properties of perfluorinated chemicals have made them desirable for extensive use in fabric, leather, and apparel treatment, in protection of food packaging and paper products, and in fire-extinguishing foam and insecticides. The inertness and great heat stability of these chemicals have also extended their industrial use.
PFCs have been shown to be persistent, to biomagnify, and to be transported into remote regions (Giesy and Kannan 2001; Smithwick et al. 2005; Tomy et al. 2004). They are potentially toxic for the environment and humans (3M 2003; Kennedy et al. 2004). Several studies have reported the increase of PFC levels in humans and wildlife up to the late 1990s (Harada et al. 2004; Holmström et al. 2004; Olsen et al. 2005b). Recent efforts by authorities and manufacturing companies to phase out the production and reduce emissions of PFCs has not yet been observed as declining environmental concentrations.
Prenatal as well as postnatal toxicity of PFOS was observed in rat and mouse, including increased liver weights, growth lags, and delayed development (Lau et al. 2003; Thibodeaux et al. 2003). Reduced weight gain and delayed development were also observed for perfluorooctanoic acid (PFOA) (Lau et al. 2004). However, assessment of risks for humans by extrapolating animal data must take into consideration several unknown pharmacokinetic parameters, such as species-specific clearance (Hanhijärvi et al. 1982; Organisation for Economic Co-operation and Development 2002).
Prenatal exposure and transfer of several PFCs through lactation has been confirmed for humans. Cord blood concentrations of PFOS were on average three times lower (1.6–5.3 ng/mL) than the corresponding maternal blood concentration from nonoccupational-exposed Japanese women (Inoue et al. 2004). A study on two human milk samples from the United States was the first to show the presence of PFCs in human milk (Kuklenyik et al. 2004). Recently So et al. (2006) reported that low levels (10–592.6 pg/mL) of PFOS, PFOA, perfluorohexane sulfonate (PFHxS), and perfluorononanoic acid (PFNA) were found in human milk of women living in China. There is to our knowledge no information about the degree of maternal PFCs transferred to the infant through lactation. A cross-fostering study on Sprague-Dawley rats indicated that PFOS is transferred to pups through lactation with a milk concentration 10–100 times lower than the maternal serum concentration (Kuklenyik et al. 2004). The concentration of PFOA in rat’s milk was estimated to be 10 times lower than the concentration in maternal plasma (Hinderliter et al. 2005). The specific transfer mechanisms are however not clear.
The objective of this study was to elucidate the relationship between maternal PFC serum levels and breast milk levels to better understand the lactational transfer of PFCs. For this purpose, matched individual milk and serum samples from 12 Swedish primiparous women were collected in 2004. In addition, the trend in Swedish breast milk PFC concentrations between 1996 and 2004 is also reported. This is the first report on the relationship of PFCs in human maternal serum and milk and the first temporal trend in breast milk PFC levels. Included in this study were four perfluoroalkylsulfonates (C4, C6, C8, C10), one polyfluorinated sulfonate (C8), seven perfluoroalkylcarboxylates (C6, C8, C9, C10, C11, C12, C14) and perfluorooctanesulfonamide (PFOSA).
Materials and Methods
Samples
Individual milk and serum samples from 12 women in Uppsala, Sweden, were collected in 2004. Milk samples from 25–90 women were collected each year between 1996 and 2004 and pooled into an annual composite sample. Donors originated from four regions in Sweden (Uppsala, Lund, Göteborg, Lycksele). All samples were from primiparous women and were collected in glass bottles during the third week after delivery and stored in plastic containers at −20°C. A summary of the sample information, including age of donors and number of pooled samples, is given in Table 1. The local ethics committee approved the design of this study, and informed consent was obtained from the study participants.
Table 1.
Overview of milk and serum samples, collected from primiparous women in Sweden.
| Region | Year of collection | No.a | Age (years)b |
|---|---|---|---|
| Composite milk | |||
| Uppsala | 1996 | 25 | 29 (21–41) |
| Uppsala | 1997 | 69 | 28 (21–38) |
| Uppsala | 1998 | 90 | 29 (22–37) |
| Uppsala | 1999 | 23 | 28 (22–36) |
| Uppsala | 2000 | 30 | 30 (21–37) |
| Göteborg | 2001 | 37 | 30 (19–40) |
| Uppsala | 2002 | 31 | 30 (24–37) |
| Lund | 2003 | 37 | 29 (25–39) |
| Lycksele | 2003–2004 | 39 | 27 (20–36) |
| Individual milk and serum | |||
| Uppsala | 2004 | — | 29 |
| Uppsala | 2004 | — | 24 |
| Uppsala | 2004 | — | 29 |
| Uppsala | 2004 | — | 29 |
| Uppsala | 2004 | — | 28 |
| Uppsala | 2004 | — | 30 |
| Uppsala | 2004 | — | 31 |
| Uppsala | 2004 | — | 27 |
| Uppsala | 2004 | — | 33 |
| Uppsala | 2004 | — | 28 |
| Uppsala | 2004 | — | 22 |
| Uppsala | 2004 | — | 29 |
Number of individual samples in the composite sample.
Median (minimum–maximum).
Chemicals
We purchased ammonium acetate [> 99%, for high-performance liquid chromatography (HPLC)] from Fluka (Steinheim, Germany), formic acid (98–100%) from Scharlau (Barcelona, Spain), and methanol (HPLC) from Labscan (Dublin, Ireland). All water used was laboratory-produced ultrapure water. Ammonium hydroxide (25% in water) and sodium acetate were purchased from Merck (Darmstadt, Germany). Perfluorobutanesulfonate (PFBuS) tetrabutylammonium salt (> 98%), PFOS potassium salt (> 98%), perfluorodecanoic acid (PFDA; > 97%), and perfluorohexanoic acid (PFHxA; > 97%) were purchased from Fluka. Perfluoroheptanoic acid (PFHpA; 99%), PFNA (97%), PFOA (96%), perfluorodecanesulfonate (PFDS) ammonium salt [25% weight in 2-butoxyethanol (37%) in water], perfluoroundecanoic acid (PFUnDA; 95%), and perfluorotetradecanoic acid (PFTDA; 97%) were purchased from Aldrich (Steinheim, Germany, and Milwaukee, WI, USA). PFOSA (97%) and 7H-PFHpA (98%) were purchased from ABCR (Karlsruhe, Germany). 1H,1H,2H,2H-PFOS (THPFOS, purity unknown), and PFHxS (98%) were purchased from Interchim (Montlucon, France). 13C4-Labeled PFOA, 13C4-labeled PFOS, and 13C5-labeled PFNA were from Wellington Laboratories (Guelph, Ontario, Canada).
Extraction
The serum and milk samples were extracted using weak anion exchange, solid-phase extraction (Waters Oasis WAX, Milford, MA, USA) based on the method by Taniyasu and colleagues (Taniyasu et al. 2005). Internal standards (13C4-PFOA and 13C4-PFOS) and 2 mL formic acid/water (1:1) were added to 1 mL milk and 0.5 mL serum. The solution was sonicated for 15 min and centrifuged at 10,000 × g for 30 min. The supernatant was extracted and the perfluorinated compounds were eluted with 1 mL 2% ammonium hydroxide in methanol, after washing the column with 2 mL sodium acetate buffer solution, pH 4, and 2 mL 40% methanol in water. Sodium acetate buffer was not used for the serum samples. The final volume for the serum extracts was 500 μL. Milk extracts were further evaporated to 30 μL, and 20 μL 2-mM ammonium acetate in water was added. Finally, filtration through a Microcon YM-3 centrifugal filter (Millipore, Billerica, MA, USA) was conducted at 14,000 × g for 30 min. Performance standards, 13C5-PFNA and 7H-PFHpA, were added to both milk and serum extracts immediately before injection.
Analysis
We performed the analysis using an Agilent 1100 HPLC system coupled to an Agilent 1100 mass spectrometric detector (Agilent, Waldbronn, Germany) with an atmospheric electrospray interface operating in negative ion mode. Separation was performed on a Discovery HS C18 (50 mm length, 2.1 mm inner diameter, 3 μm particles, 120 Å pore size) column with a guard column of the same material (20 mm length, 2.1 mm inner diameter, 3 μm particles, 120 Å pore size) (Supelco, Bellefonte PA, USA). Both columns were kept at 40°C. An extra guard column (HyperCarb graphitic carbon, 4 mm length, 10 mm inner diameter, 5 μm particle size; Termo Hypersil-Keystone, Bellefonte PA, USA) was inserted between the pump and injector to remove any fluorochemicals originating from the HPLC system. Injection volume was 10 μL and the flow rate was set to 300 μL/min. The mobile phases consisted of 2 mM ammonium acetate in methanol and 2 mM ammonium acetate in water. HPLC gradient and MS settings have been described in detail elsewhere (Karrman et al. 2005).
Quality assurance
Quantification was performed using the internal standard method with standards dissolved in 35% methanol in water. We used 13C4-PFOS as internal standard for the sulfonates and PFOSA, and 13C4-PFOA for the carboxylates. We used 13C5-PFNA and 7H-PFHpA to monitor the recovery of the internal standards. The recovery was on average 67%, within 50–130% for 78% of all milk samples and within 84–97% for all serum samples. An overview of the method performance is given in Table 2. Recoveries were evaluated by three or five replicate fortifications to a low-contaminated serum sample and a breast milk sample containing PFCs below the detection limit. Average recoveries were > 50% for all compounds except for PFOSA, PFDA, PFUnDA, and PFDoDA (34–47%) and the coefficient of variation (CV) was 2–27% for multiple determinations. Containers used for storage of milk and serum samples were extracted with methanol and did not show traces of the target compounds. Procedural blank trace levels were detected for PFOA, PFOS, and PFNA (Table 3). In the case of blank levels, the mean blank signal plus 3 SDs of multiple blank injections were subtracted from the calculated concentrations in the samples. A blank corrected concentration was reported provided that the blank level was ≤50% of the uncorrected concentration. Detection limits for serum and breast milk were 0.1–1.1 ng/mL and 0.005–0.209 ng/mL, respectively. The selectivity of the mass spectrometry (MS) analysis was verified by triple quadrupole mass spectrometry (MS/MS) analysis. All breast milk samples (1 mL) were extracted in duplicates. The first sample extract was evaporated to 50 μL and injected on the LC-MS system (10 μL). The second extract was kept at 500 μL, of which 200 μL was injected on a column-switching LC system connected to a triple quadrupole MS system (Micromass QuattroII, Altrincham, UK). Further quality assurance was taken by successful participation in the first interlaboratory study on PFCs (Van Leeuwen et al. 2006).
Table 2.
Performance of the methods for extracting human serum and milk samples.
| Percent of recovery (CV)
|
Detection limit (ng/mL)a |
Blank concentration (ng/mL)b |
||||
|---|---|---|---|---|---|---|
| Compound | Serum (n = 5) | Milk (n = 3) | Serum | Milk | Serum | Milk |
| PFBuS | 85 (2) | 79 (4) | 0.7 | 0.05 | — | — |
| PFHxS | 89 (3) | 81 (3) | 0.2 | 0.01 | — | — |
| PFOS | 82 (5) | 83 (3) | 0.2 | 0.005 | — | 0.050 |
| THPFOS | 53 (4) | 51 (5) | 1.1 | 0.07 | — | — |
| PFDS | 39 (9) | 72 (4) | 0.2 | 0.04 | — | 0.084 |
| PFHxA | 82 (3) | 80 (2) | 0.7 | 0.1 | — | — |
| PFHpA | 82 (2) | 84 (4) | 0.3 | 0.1 | — | — |
| PFOA | 89 (2) | 82 (4) | 0.4 | 0.01 | 0.5 | 0.209 |
| PFNA | 95 (2) | 77 (2) | 0.2 | 0.005 | — | — |
| PFDA | 56 (5) | 43 (27) | 0.1 | 0.008 | — | 0.014 |
| PFUnDA | 47 (7) | 38 (3) | 0.2 | 0.005 | — | 0.008 |
| PFDoDA | 41 (11) | 39 (5) | 0.5 | 0.005 | — | — |
| PFOSA | 47 (4) | 34 (0) | 0.1 | 0.007 | — | — |
Abbreviations: —, below the detection limit; CV, coefficent of variation. Values for detection limit and blank concentration are from multiple experiments.
Not including eventual blank concentrations.
Mean ± 3 SD of detected signal from injecting 10 μL of a procedural blank.
Table 3.
Levels (ng/mL) of seven PFCs in matched milk and serum samples from 12 primiparous Swedish women, 2004.
| PFHxS | PFOS | PFOSA | PFOA | PFNA | PFDA | PFUnDA | |
|---|---|---|---|---|---|---|---|
| Serum | |||||||
| No. > LOD | 12 | 12 | 9 | 12 | 12 | 12 | 12 |
| Range | 1.8–11.8 | 8.2–48.0 | < 0.10–0.49 | 2.4–5.3 | 0.43–2.5 | 0.27–1.8 | 0.20–1.5 |
| Mean | 4.7 | 20.7 | 0.24 | 3.8 | 0.80 | 0.53 | 0.40 |
| SD | 2.9 | 10.5 | 0.16 | 1.0 | 0.55 | 0.41 | 0.35 |
| Median | 4.0 | 18.7 | 0.19 | 3.8 | 0.63 | 0.43 | 0.28 |
| Milk | |||||||
| No. > LOD | 12 | 12 | 8 | 1a | 2 | 0 | 0 |
| Range | 0.031–0.172 | 0.060–0.470 | < 0.007–0.030 | < 0.209b–0.492 | < 0.005–0.020 | < 0.008 | < 0.005 |
| Mean | 0.085 | 0.201 | 0.013 | NA | 0.017 | NA | NA |
| SD | 0.047 | 0.117 | 0.009 | NA | NA | NA | NA |
| Median | 0.070 | 0.166 | 0.010 | NA | NA | NA | NA |
| M:S | 0.02:1 | 0.01:1 | 0.07:1 | 0.12:1 | 0.01:1 | NA | NA |
| CV | 28 | 38 | 67 | NA | 52 | NA | NA |
NA, not applicable.
Eleven additional samples were above the detection limit (0.01 ng/mL) but the blank level was > 50% of the detected concentrations (blank level 0.209 ng/mL).
Blank level.
Results
A summary of the results of 12 individual matched milk and serum samples is given in Table 3. Highest mean serum concentration was obtained for PFOS (20.7 ng/mL) followed by PFHxS (4.7 ng/mL), PFOA (3.8 ng/mL), PFNA (0.80 ng/mL), PFDA (0.53 ng/mL), PFUnDA (0.40 ng/mL), and PFOSA (0.24 ng/mL). PFDS was detected in only one serum sample (0.33 ng/mL). Of the eight PFCs found in the serum samples, five were detected in the matched milk samples at the current detection limits. PFOS and PFHxS were detected in all milk samples at mean concentrations of 0.201 ng/mL and 0.085 ng/mL, respectively. PFOSA was detected in eight milk samples with a mean concentration of 0.013 ng/mL, and PFNA was detected in two milk samples (0.020 and 0.014 ng/mL). Similar PFC occurrence and levels were found in the milk composite samples collected during the 8 years between 1996 and 2004 (Table 4).
Table 4.
Levels (ng/mL) of 5 PFCs in composite milk samples from primiparous Swedish women.
| Region | Year of collection | PFHxS | PFOA | PFNA | PFOS | PFOSA |
|---|---|---|---|---|---|---|
| Uppsala | 1996 | 0.037 | < 0.209a | 0.028 | 0.209 | < 0.007 |
| Uppsala | 1997 | 0.030 | < 0.209a | < 0.005 | 0.207 | < 0.007 |
| Uppsala | 1998 | 0.040 | < 0.209a | < 0.005 | 0.219 | < 0.007 |
| Uppsala | 1999 | 0.044 | < 0.209a | < 0.005 | 0.213 | < 0.007 |
| Uppsala | 2000 | 0.028 | < 0.209a | 0.019 | 0.191 | < 0.007 |
| Göteborg | 2001 | 0.028 | < 0.209a | < 0.005 | 0.258 | < 0.007 |
| Uppsala | 2002 | 0.051 | < 0.209a | < 0.005 | 0.194 | < 0.007 |
| Lund | 2003 | 0.025 | < 0.209 | < 0.005 | 0.153 | < 0.007 |
| Lycksele | 2003–2004 | 0.016 | < 0.209 | 0.020 | 0.123 | < 0.007 |
Levels were above the detection limit (0.01 ng/mL) but the blank level was > 50% of the detected concentrations (blank level 0.209 ng/mL).
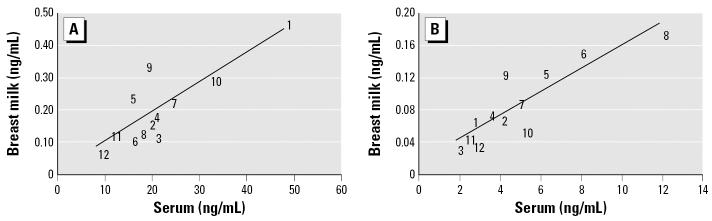
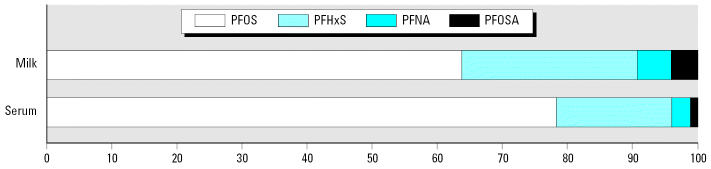
Milk levels were lower compared with the matched serum levels on a volume basis (nanograms per milliliter). The mean ratio between milk and serum (M:S) concentration was 0.01:1 for PFOS, 0.02:1 for PFHxS, and 0.07:1 for PFOSA (Table 3). The M:S ratios for PFOA and PFNA are uncertain because only one and two milk samples, respectively, contained levels above the detection limit. Simple regression analysis and the Spearman’s correlation test of the matched serum and milk samples show a significant association (r2 = 0.7–0.8, p < 0.05) between levels of PFOS and PFHxS in serum and milk (Figure 1). The percentage contribution of each of PFHxS, PFOS, PFOSA, and PFNA to the total concentration in serum and milk is given in Figure 2.
Figure 1.
Scatterplot and regression analysis of levels in matched serum and milk samples from 12 Swedish primiparous women (numbered 1–12), 2004. (A) PFOS (M:S ratio, 0.01:1; CV, 38%; y = 0.0092x + 0.0104; r2 = 0.6868). (B) PFOA (M:S ratio, 0.02:1; CV, 28%; y = 0.0144x + 0.0178; r2 = 0.7707).
Figure 2.
The average contribution (%) of PFOS, PFHxS, PFNA, and PFOSA to the total concentration in 12 matched milk and serum samples from primiparous women in Sweden, 2004.
The limits of detection (LOD) in human milk were between 0.005 and 0.010 ng/mL, except for PFHxA and PFHpA, which were an order of magnitude higher (0.1 ng/mL) (Table 2). A relatively high blank level was obtained for PFOA (0.209 ng/mL). PFOA is reported in only one milk sample as a consequence of the high blank level and the quantification criteria.
Discussion
The serum levels in the present study are similar to or lower than the levels found in a previous study on 17 Swedish human plasma samples collected in 1998–2000 from men and women (Karrman et al. 2004). The Swedish PFOS and PFOA blood levels are similar to levels in, for example, Canada, Australia, and some less-industrialized regions in Japan, but somewhat lower than reported blood levels in the United States (Harada et al. 2004; Karrman et al. 2006a; Kubwabo et al. 2004; Olsen et al. 2003).
Only one study originating from China has previously reported levels of several PFCs in human milk. The Swedish levels are comparable to human milk from China except for those for PFOSA, which was not included in the Chinese study (So et al. 2006). In addition to detected PFCs in the present study, PFHpA, PFDA, and PFUnDA were also found in human milk from China.
PFOSA was frequently detected in the milk samples, unlike PFNA, PFDA and PFUnDA, even though the latter were detected at higher concentrations in the serum samples. This is most likely caused by the fact that PFOSA concentrations in plasma have been found to be only about 20% of the whole blood concentration on a volume basis (Karrman et al. 2006b). The total blood concentration of PFOSA available for excretion to milk is therefore about five times higher than the measured concentration in serum. The M:S ratio for PFOSA should therefore be close to that of PFOS if nearly all PFOSA were distributed to serum. The serum and milk pattern suggests that PFHxS is excreted to milk in a higher degree than PFOS and PFOSA. A preferential excretion of shorter, less hydrophobic PFCs is a possible explanation of the observed pattern, but could not be concluded in the present study because of the limited number of matched milk and serum samples.
The presented linear relationship between serum and milk levels suggests a partitioning process, which can be predicted from the PFC blood concentration on a volume basis. The steeper slope of PFHxS demonstrates the higher partition than PFOS to milk (Figure 1). The association between milk and serum concentrations could not be seen for PFOSA (r2 < 0.1). The PFOSA ratio between milk and serum can be influenced by several parameters. First, it has been suggested that PFOSA can degrade to PFOS in biologic systems (Tomy et al. 2003), which might affect the ratio between milk and serum. Second, PFOSA is partly lost during the separation of the red blood cells, which makes serum a poor matrix for determining PFOSA blood concentrations. Finally, relatively more milk and serum samples had levels of PFOSA close to the detection limit.
For more fat-soluble, persistent organohalogens, the levels in blood and milk are about the same when calculated on a fat basis and with a steady state assumption. On a volume basis, the ratio of lipophilic compounds in whole blood and milk is approximately 1:10, because of the higher lipid content in milk than in blood (Jensen and Slorach 1991). The lactational transfer of PFCs may be more similar to that of heavy metals. For example, the concentration of lead in milk has been found to be 5–10 times lower than that in blood (Jensen and Slorach 1991). Perfluorinated acids are generally believed to bind to serum albumin (Jones et al. 2003). It has been demonstrated that serum albumin in plasma has a large binding capacity for PFOA (6–9 binding sites per molecule and millimolar concentration in plasma) and the free fraction of PFOA in plasma was estimated to be < 5% (Han et al. 2003). The reason for the relatively higher PFC concentration in human serum than in milk is unknown.
Excretion of PFCs into milk may be accomplished by two ways that have been identified as transport mechanisms for chemical contaminants: binding to milk protein (protein content ~ 1 g/100 mL milk) or to the surface of fat (fat content ~ 4 g/100 mL milk) (Jensen and Slorach 1991). The fat content in milk fluctuates but does not vary significantly during the course of lactation, unlike the total protein content, which was shown to decrease rapidly during the first month of lactation. The serum albumin content of milk was, however, stable during a 6.5-month period of lactation (Lönnerdal et al. 1976). Assessing the amount of PFCs transferred and adsorbed by an infant during the course of lactation involves several assumptions—for instance, the variation of PFC concentration in milk with time and the uptake efficiency of PFCs from milk by the infant. The total mean PFC concentration of all detected compounds in the present study was 32 ng/mL in serum and 0.34 ng/mL in milk. Hypothetically, a lactation of 600 mL/day and 100% uptake would produce an exposure burden for an infant (and maternal excretion) of 203 ng PFCs per day, corresponding to 34 μg PFCs after 6 months, given a constant PFC concentration in milk during 6 months. A risk assessment is unfeasible because of the lack of human hazard assessment of each of the detected PFCs and of relevant reference intake levels or concentrations to compare with. However, So et al. (2006) used a reference dose (25 ng/kg/day) for PFOS estimated by the Environmental Working Group, based on the end point of increase in mammary fibroadenomas in a rat chronic toxicity study (Thayer and Houlihan 2002). Using the same assumptions (milk consumption 600 g/day, body weight 7 kg), two milk samples with the highest PFOS concentration in our study (0.465 and 0.337 ng/mL) exceed the reference dose and would therefore constitute a risk to the infant. However, there are several uncertainties that need clarification before any conclusions can be made.
This study contributes to PFC exposure risk assessments for infants, and the evaluation of lactation as an exposure pathway as well as a way for maternal excretion. Several studies indicate that females have lower blood concentrations of several PFCs than do males (Calafat et al. 2006a; Karrman et al. 2006a; Olsen et al. 2003). Elimination through lactation could be one explanation for this observation. However, a sex difference was observed also for 2- to 12-year-old children in the United States (Olsen et al. 2004).
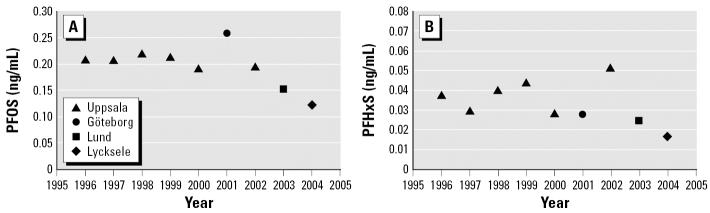
PFOS and PFHxS were detected in composite milk samples collected each year between 1996 and 2003–2004 from four different regions in Sweden (Table 4). The variation of PFOS and PFHxS in the composite samples is remarkably small (a total variation of 20% and 32% CV, respectively), indicating that milk levels of PFOS and PFHxS have been constant in the last 8 years. Consequently, no clear temporal trend could be distinguished (Figure 3). However, the samples from 2001, 2003, and 2003–2004 were from regions different from the rest of the samples. Possible regional differences in human PFC levels in Sweden remain yet to be established. PFOS has been present in the Swedish environment at least since 1968, and the levels increased dramatically up to 1997 in guillemot eggs (Holmström et al. 2004). PFOS-related products were imported in Sweden until 2002 and will probably be used for a long period of time [KEMI (Swedish Chemicals Agency) 2004]. The global production of perfluorooctanesulfonyl fluoride started to decrease in 2001 after the phase-out decision by the major producer 3M (3M 2000). A possible effect of the actions taken by governments and producers in terms of declining environmental and human concentrations needs to be monitored for several years to come because of the persistence of PFCs [PFOS half-life is approximately 5 years in humans (Olsen et al. 2005a)].
Figure 3.
Temporal trend for (A) PFOS and (B) PFHxS in human composite milk samples from different regions in Sweden, 1996–2004.
The relatively low levels of PFCs present in the human milk samples challenged the analysis. By reducing the volume of the milk sample extracts by a factor of 100, required detection limits were achieved. As a consequence, traces in the procedural blanks were seen for several of the compounds monitored (Table 2). A confident quantification of PFOA in the milk samples was hampered by a high procedural blank contamination. PFOA is usually the second highest PFC found in human blood, except in Korea where PFOA levels have been reported to exceed those of PFOS (Kannan et al. 2004). PFOA contributed up to 36% of the total PFC content in human milk from China (So et al. 2006). The selectivity of the single quadrupole MS method was successfully verified with triple quadrupole MS/MS analysis. Qualitative comparison indicated that MS/MS analysis demonstrated on average 50% higher concentrations compared with the single quadrupole MS analysis. However, different preconcentration methods were used for the different instruments, and the differences seen between the methods can be multifactorial.
Conclusions
The PFC level in human milk are about 1% of the corresponding level in serum. There is an indication that elimination of PFCs through lactation is compound-dependent and partitioning of PFCs into milk seems to relate to the concentration in maternal blood. A trend of PFC concentrations in milk between 1996 and 2004 could not be observed in the present study. Lactation is a considerable source of PFC exposure for infants. The present study indicates that approximately 200 ng PFCs per day may be transferred from a lactating mother to the infant. Reference concentrations as well as information on the infant’s uptake and excretion of PFCs during the lactation period are urgently needed for a full risk assessment. The ubiquitous presence and levels of PFOS in human milk justifies further monitoring of this class of contaminants in human milk worldwide.
Correction
In the manuscript originally published online, in Table 2, some of the values for the blank concentrations of serum and milk were incorrect. Figure 1B describes PFOA, not PFHxS. These errors have been corrected here.
Footnotes
K. Holmström and M. McLachlan (Stockholm University) are kindly acknowledged for the assistance with the triple quadrupole mass spectrometry analysis.
The study was financially supported by the Swedish Environmental Protection Agency (HÄMI). The Cancer and Allergy foundation is also acknowledged for their support.
References
- 3M 2000. Phase-out Plan for PFOS-based Products. U.S. Environmental Protection Agency Public Docket AR226–0588.
- 3M 2003. Environmental and Health Assessment of Perfluorooctane Sulfonic Acid and Its Salts. U.S. Environmental Protection Agency Public Docket AR226–1486.
- Calafat AM, Kuklenyik Z, Caudill SP, Reidy JA, Needham LL. Perfluorochemicals in pooled serum samples from United States residents in 2001 and 2002. Environ Sci Technol. 2006a;40:2128–2134. doi: 10.1021/es0517973. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Needham LL, Kuklenyik Z, Reidy JA, Tully JS, Aguilar-Villalobos M, et al. Perfluorinated chemicals in selected residents of the American continent. Chemosphere. 2006b;63:490–496. doi: 10.1016/j.chemosphere.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA, Jepson GW. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem Res Toxicol. 2003;16:775–781. doi: 10.1021/tx034005w. [DOI] [PubMed] [Google Scholar]
- Hanhijärvi H, Ophaug RH, Singer L. The sex-related difference in perfluorooctanoate excretion in the rat. Proc Soc Exp Biol Med. 1982;171:50–55. doi: 10.3181/00379727-171-41476. [DOI] [PubMed] [Google Scholar]
- Harada K, Saito N, Inoue K, Yoshinaga T, Watanabe T, Sasaki S, et al. The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate and perfluorooctanoate in human serum over the last 25 years. J Occup Health. 2004;46:141–147. doi: 10.1539/joh.46.141. [DOI] [PubMed] [Google Scholar]
- Hinderliter PM, Mylchreest E, Gannon SA, Butenhoff JL, Kennedy GL. Perfluorooctanoate: placental and lactational transport pharmacokinetics in rats. Toxicology. 2005;211:139–148. doi: 10.1016/j.tox.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Holmström KE, Järnberg U, Bignert A. Temporal trends of PFOS and PFOA in guillemot eggs from the Baltic Sea, 1968–2003. Environ Sci Technol. 2004;39:80–84. doi: 10.1021/es049257d. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima AU, et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in susceptible population during pregnancy. Environ Health Perspect. 2004;112:1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Slorach SA. 1991. Chemical Contaminants in Human Milk. Boca Raton, FL:CRC Press.
- Jones PD, Hu W, de Coen W, Newsted JL, Giesy JP. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem. 2003;22:2639–2649. doi: 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillman G, Kumar KS, Loganathan BG, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Karrman A, Mueller JF, van Bavel B, Harden F, Toms L-ML, Lindström G. Levels of 12 perfluorinated chemicals in pooled Australian serum collected 2002–2003 in relation to age, gender and region. Environ Sci Technol. 2006a;40:3742–3748. doi: 10.1021/es060301u. [DOI] [PubMed] [Google Scholar]
- Karrman A, van Bavel B, Järnberg U, Hardell L, Lindström G. Perfluorinated chemicals in relation to other persistent organic pollutants in human blood. Chemosphere. 2006b;64:1582–1591. doi: 10.1016/j.chemosphere.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Karrman A, van Bavel B, Järnberg U, Hardell L, Lindström G. 2004. Perfluoroalkylated compounds in whole blood and plasma from the Swedish population. Naturvårdsverket, häslorelaterad miljöövervakning (Swedish EPA). Available: http://www.naturvardsverket.se/dokument/mo/modok/export/pfos_blod.pdf [accessed 25 October 2006].
- Karrman A, van Bavel B, Järnberg U, Hardell L, Lindström G. Development of a solid-phase extraction-HPLC/single quadrupole MS method for quantification of perfluorochemicals in whole blood. Anal Chem. 2005;77:864–870. doi: 10.1021/ac049023c. [DOI] [PubMed] [Google Scholar]
- KEMI 2004. PFOS-relaterade ämnen: strategi för utfasning [in Swedish]. Stockholm:Swedish Chemicals Agency. Available: http://www.kemi.se/upload/Trycksaker/Pdf/Rapporter/Rapport3_04.pdf [accessed 15 May 2006].
- Kennedy GL, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AM, Perkins RG, et al. The toxicology of perfluorooctanoate. Crit Rev Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- Kubwabo C, Vais N, Benoit FM. A pilot study on the determination of perfluorooctanesulfonate and other perfluorinated compounds in blood of Canadians. J Environ Monitor. 2004;6:540–545. doi: 10.1039/b314085g. [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z, Reich JA, Tully LL, Needham LL, Calafat AM. Automated solid-phase extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ Sci Technol. 2004;38:3698–3704. doi: 10.1021/es040332u. [DOI] [PubMed] [Google Scholar]
- Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198:231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II. Postnatal evaluation. Toxicol Sci. 2003;74:382–392. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B, Forsum E, Hambraeus L. A longitudinal study of the protein, nitrogen, and lactose contents of human milk from Swedish well-nourished mothers. Am J Clin Nutr. 1976;29:1127–1133. doi: 10.1093/ajcn/29.10.1127. [DOI] [PubMed] [Google Scholar]
- Olsen G, Ehresman D, Froehlich J, Burris J, Butenhoff J. 2005a. Evaluation of the half-life (t1/2) of elimination of perfluorooctanesulfonate (PFOS), perfluorohexanesulfonate (PFHxS) and perfluorooctanoate (PFOA) from human serum. In: International symposium on fluorinated alkyl organics in the environment. TOX017. Available: http://www.chem.utoronto.ca/symposium/fluoros/toxicology.htm [accessed 11 December 2006].
- Olsen GW, Church TR, Hansen KJ, Burris JM, Butenhoff JL, Mandel JH, et al. Quantitative evaluation of perfluorooctanesulfonate (PFOS) and other fluorochemicals in the serum of children. J Child Health. 2004;2:53–76. [Google Scholar]
- Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, et al. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross blood donors. Environ Health Perspect. 2003;111:1892–1901. doi: 10.1289/ehp.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Huang H-Y, Helzlsouer KJ, Hansen KJ, Butenhoff JL, Mandel JH. Historical comparison of perfluorooctanesulfonate, perfluorooctanoate, and other fluorochemicals in human blood. Environ Health Perspect. 2005b;113:539–545. doi: 10.1289/ehp.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development 2002. Draft Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts: Complete Assessment. ENV/JM/RD(2002)17/FINAL. Paris:Organisation for Economic Co-operation and Development.
- Smithwick M, Mabury SA, Solomon KR, Sonne C, Martin JW, Born EW, et al. Circumpolar study of perfluoroalkyl contaminants in polar bears (Ursus maritimus) Environ Sci Technol. 2005;39:5517–5523. doi: 10.1021/es048309w. [DOI] [PubMed] [Google Scholar]
- So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, et al. Health risks in infants associated with exposure to perfluorinated compunds in human breast milk from Zhoushan, China. Environ Sci Technol. 2006;40:2924–2929. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- Taniyasu S, Kannan K, Ka So M, Gulkowska A, Sinclair E, Okazawa T, et al. Analysis of fluorotelomer alcohols, fluortelomer acids and short- and long-chain perfluorinated acds in water and biota. J Chrom A. 2005;1093:89–97. doi: 10.1016/j.chroma.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Thayer K, Houlihan J. 2002. Perfluorinated chemicals: Justification for Inclusion of This Chemical Class in the National Report on Human Exposure to Environmental Chemicals. Washington, DC:Environmental Working Group. Available: http://www.ewg.org/issues_content/pfcs/pdf/EWG_CDC_petition_2002.pdf [accessed 29 June 2006].
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, et al. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I. Maternal and prenatal evaluations. Toxicol Sci. 2003;74:369–381. doi: 10.1093/toxsci/kfg121. [DOI] [PubMed] [Google Scholar]
- Tomy GT, Budakowski WR, Halldorson T, Helm PA, Stern GA, Friesen K, et al. Fluorinated organic compounds in an eastern arctic marine food web. Environ Sci Technol. 2004;38:6475–6481. doi: 10.1021/es049620g. [DOI] [PubMed] [Google Scholar]
- Tomy GT, Tittlemier SA, Palace VP, Budakowski WR, Braekevelt E, Brinkworth L, et al. Biotransformation of N-ethyl perfluorooctanesulfonamide by rainbow trout (Onchorhynchus mykiss) liver microsomes. Environ Sci Technol. 2003;38:758–763. doi: 10.1021/es034550j. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen SPJ, Karrman A, van Bavel B, de Boer J, Lindström G.2006Struggle for quality in determination of perfluorinated contaminants in environmental and human samples Environ Sci Technol 10.1021/es061052c [Online 11 November 2006]. [DOI] [PubMed] [Google Scholar]
- Yeung LW, So MK, Jiang G, Taniyasu S, Yamashita N, Song M, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood samples from China. Environ Sci Technol. 2006;40:715–720. doi: 10.1021/es052067y. [DOI] [PubMed] [Google Scholar]